Abstract
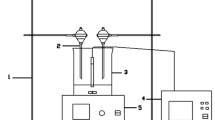
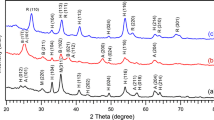
The photocatalytic activity of new hollandite compounds was investigated for the oxidative decomposition of trichloro ethylene (ClHC=CCl2) and the selecive reduction of nitrate ion (NO -3 ) with methanol in water under UV irradiation. The ClHC=CCl2 and the NO -3 in water are hazardous chemicals to humans. Social interest is put on the development of effective processes to remove these nuisances. The hollandite compounds have a one-dimensional tunnel structure and are well known as alkali ion conductors. In this study, their distinctive catalytic properties in gas phase under heating were applied to photocatalytic reactions. The hollandite catalysts which were prepared by the powder mixing method or sol-gel method had activities for the oxidation of ClHC=CCl2 and the selective reduction of NO -3 with methanol in water under UV irradiation. The selective reduction of NO -3 with a reducing agent in the presence of dissolved oxygen in water would be the new way which has not been reported for ordinary photocatalysts such as titanium dioxide (TiO2). Therefore, it was expected that this type of compound had a possibility as a new type of photocatalyst.
Similar content being viewed by others
REFERENCES
M. A. Fox and M. T. Dulay, Chem. Rev. 93, 341–357 (1993).
P. V. Kamat, Chem. Rev. 93, 267–300 (1993).
A. Fujishima and K. Honda, Bull. Chem. Soc. Jpn., 44, 1148–1150 (1971).
A. Fujishima and K. Honda, Nature, 238, 37–38 (1972).
F. Mollers, H. J. Tolle, and R. Memming, J. Electrochem. Soc., 121, 1160–1167 (1974).
K. L. Hardee and A. J. Bard, J. Electrochem. Soc., 122, 739–742 (1975).
B. Kraeutler and A. J. Bard, J. Am. Chem. Soc., 100, 5985–5992 (1978).
W. W. Dunn, Y. Aikawa, and A. J. Bard, J. Am. Chem. Soc., 103, 3456–3459 (1981).
D. Duonghong, E. Borgarello, and M. Gratzel, J. Am. Chem. Soc., 103, 4685–4690 (1981).
K. Hashimoto and A. Fujishima, Catalysis, 36, 524–530 (1994).
K. Hashimoto and A. Fujishima, Yosui and Haisui, 36, 851–857 (1994).
M. Watanabe, T. Mori, S. Yamauchi, and H. Yamamura, Solid State Ionics, 102, 376–381 (1994).
T. Mori, S. Yamauchi, H. Yamamura, and M. Watanabe, Appl. Catal. A, 129, L1–L7 (1995).
T. Mori, S. Yamauchi, H. Yamamura, and M. Watanabe, Proc. International Workshop on Interface of Ceramic Materials, ÅgKey Engineering Materials Vols. 111–112Åh, 71–82 (1995).
T. Mori, S. Yamauchi, H. Yamamura, and M. Watanabe, J. Mater. Sci., 31, 1469–1473 (1996).
A. Bystrom and A. M. Bystrom, Acta. Cryst., 3, 146 (1950).
Y. Fujiki, S. Takenouchi, Y. Onoda, M. Watanabe, S. Yoshikado, T. Ohachi, and I. Taniguchi, Solid State Ionics, 25, 131–137 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mori, T., Suzuki, J., Fujimoto, K. et al. Photocatalytic Decomposition of Trichloro Ethylene and Nitrate Ion in Water on Hollandite-Type Catalysts. Journal of Materials Synthesis and Processing 6, 329–333 (1998). https://doi.org/10.1023/A:1022651210732
Issue Date:
DOI: https://doi.org/10.1023/A:1022651210732