Abstract
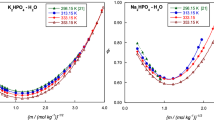
The enthalpies of dilution of aqueous solutions of HCl, H3PO4, NaOH, NaH2PO4, Na2HPO4 and Na3PO4 in the molality range 0.1 to 1.0 mole-kg−1 have been determined at 30°C. The relative apparent molal enthalpies φL of HCl, NaOH, NaH2PO4 and Na2HPO4 have been determined with the aid of an extended form of the Debye-Hückel limiting law. The relative apparent molal enthalpies for Na3PO4 solutions have been corrected for hydrolysis. A value of ΔH oH =9525±150 cal-mole−1 was determined for the heat of hydrolysis of PO −34 . This value gives ΔH o3 =3815±150 cal-mole−1 for the ionization of H2PO −4 , which is in good agreement with the value of ΔH o3 =3500±500 cal-mole−1 determined directly by Pitzer at 25°C. The relative apparent molal enthalpies for H3PO4 solutions have been corrected for ionization. A value of ΔH o1 =−1900±150 cal-mole−1 was obtained for the heat of ionization of H3PO4 to H++H2PO −4 . This value is in good agreement with the value of ΔH o1 =−2031 cal-mole−1 at 30°C determined by Harned and Owen from the temperature coefficient of the equilibrium constant and ΔH o1 =−1950±80 cal-mole−1 at 25°C determined from calorimetry by Pitzer.
Similar content being viewed by others
References
R. S. Dietz, K. O. Emery, and F. P. Shepard,Bull. Geol. Soc. Am. 53, 815 (1942).
C. E. Roberson,Solubility implications of apatite in seawater, M.S. Thesis, University of California, San Diego (1965).
R. H. Wood and R. F. Platford,J. Solution Chem. 4, 977 (1975).
R. F. Platford,J. Chem. Eng. Data 21, 468 (1976).
K. S. Pitzer and L. F. Silvester,J. Solution Chem. 5, 269 (1976).
G. K. Ward and F. J. Millero,J. Chem. Thermodyn. 5, 591 (1973).
W. H. Leung and F. J. Millero,J. Solution Chem. 4, 145 (1975).
W. H. Leung and F. J. Millero,J. Chem. Thermodyn. 7, 1067 (1975).
F. J. Millero, L. D. Hansen, and E. V. Hoff,J. Mar. Res. 31, 21 (1973).
H. W. Jongenberger and R. H. Wood,J. Phys. Chem. 69, 4231 (1965).
G. N. Lewis and M. Randall,Thermodynamics, 2nd rev. ed., K. S. Pitzer and L. Brewer, eds. (McGraw-Hill, New York, 1961).
H. S. Harned and B. B. Owen,The Physical Chemistry of Electrolyte Solutions (Reinhold, New York, 1958).
R. A. Robinson and R. H. Stokes,Electrolyte Solutions (Butterworths, London, 1959).
L. G. Sillen and A. E. Martell,Stability Constants of Metal-Ion Complexes (The Chemical Society, London, 1964).
K. S. Pitzer and G. Mayorga,J. Phys. Chem. 77, 2300 (1973).
J. D. Hale, R. M. Izatt, and J. J. Christensen,J. Phys. Chem. 67, 2605 (1963).
C. E. Vanderzee and J. A. Swanson,J. Phys. Chem. 67, 2608 (1963).
K. S. Pitzer,J. Am. Chem. Soc. 59, 2365 (1937).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Millero, F.J., Duer, W.C., Shepard, E. et al. The enthalpies of dilution of phosphate solutions at 30°C. J Solution Chem 7, 877–889 (1978). https://doi.org/10.1007/BF00645297
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00645297