Abstract
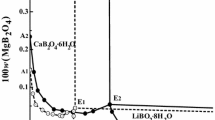
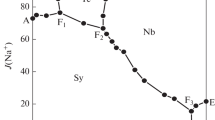
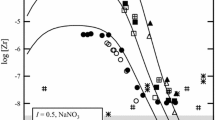
The stoichiometric solubility constant of eitelite (NaMg 0.5 CO 3 +2H+ ⇄ Na++0.5Mg2++CO 2 (g)+H 2 O, log*K Ipso =14.67±0.03 was determined at I=3 m (mol kg−1) (NaClO 4 ) and 25°C. The stability of magnesium (hydrogen-)carbonato complexes in this ionic medium was explicitely taken into account. Consequently, trace activity coefficients of free ionic species, calculated from the Pitzer model with ion-interaction parameters from the literature, were sufficient for an evaluation of the thermodynamic solubility constants and Gibbs energies of formation for eitelite (−1039.88±0.60), magnesite (−1033.60±0.40), hydromagnesite (−1174.30±0.50), nesquehonite (−1724.67±0.40), and brucite (−835.90±0.80 kJ-mol−1). The increasing solubilities of nesquehonite and eitelite at higher sodium carbonate molalities were explained by invoking a magnesium dicarbonato complex (Mg2++2CO 2−3 ⇄ Mg(CO3) 2−2 , log βz = 3.90 ± 0.08). A set of ion-interaction parameters was obtained from solubility and dissociation constants for carbonic acid in 1 to 3.5 m NaClO 4 media \((\theta _{HCO_3^ - ,ClO_4^ - } = 0.081, \theta _{CO_3^{2 - } ,ClO_4^ - } = 0.071, \psi _{{\rm N}a^ + , HCO_3^ - , ClO_4^ - } = - 0.019,\psi _{{\rm N}a^ + , CO_3^{2 - } , ClO_4^ - } = - 0.006,\lambda _{ClO_4^ - ,CO_2 } = - 0.076)\) which reproduce these constants to 0.02 units in log K. The following Pitzer parameters are consistent with the previously studied formation of magnesium (hydrogen-)carbonato complexes in 3m NaClO 4 \((\psi _{Mg^{2 + } , HCO_3^ - , ClO_4^ - } = - 0.36, \lambda _{ClO_4^ - ,MgCO_3 } = 0.081)\). The model and Gibbs functions of solid phases derived here reproduce original solubility data (−log [H+], [Mg2+] tot ) measured in perchlorate medium within experimental uncertainty.
Similar content being viewed by others
References
E. Königsberger, R. Hausner, and H. Gamsjäger,Geochim. Cosmochim. Acta 55, 3505 (1991).
K. S. Pitzer,J. Phys. Chem. 77, 268 (1973).
C. E. Harvie, N. Møller, and J. H. Weare,Geochim. Cosmochim. Acta 48, 723 (1984).
P. W. Schindler,Chimia 17, 313 (1963).
H. Gamsjäger, H. U. Stuber and P. W. Schindler,Helv. Chim. Acta 48, 723 (1965).
E. Königsberger and H. Gamsjäger,Ber. Bunsenges. Phys. Chem. 91, 785 (1987).
E. Königsberger and H. Gamsjäger,Marine Chemistry 30, 317 (1990).
J. C. Deelman,N. Jb. Miner. Mh. 10, 468 (1984).
W. F. Riesen,Thermodynamische Untersuchungen am Quaternären System Ca 2+ −Mg 2+−CO2−H2 O (PhD Thesis, Universität Bern, 1969).
G. Horn,Radex-Rundschau 1969, 439 (1969).
G. Eriksson and K. Hack,Metall. Trans. B 21 B, 1013 (1990).
F. J. C. Rossotti and H. Rossotti,The Determination of Stability Constants (McGraw-Hill, New York, 1961).
G. Biedermann and L. G. Sillén,Arkiv Kemi 5, 425 (1953).
W. Kraft,Monatsh. Chem.,98, 1978 (1967).
G. von Knorre,Z. Anorg. Allg. Chemie 34, 260 (1903).
A. Pabst,Amer. Mineral. 58, 211 (1982).
H. Gamsjäger and F. Reiterer,Environ. Int. 2, 419 (1979).
W. F. Riesen, H. Gamsjäger and P. W. Schindler,Geochim. Cosmochim. Acta 41, 1193 (1977).
G. Nilsson, T. Rengemo, and L. G. Sillén,Acta Chem. Scand. 12, 868 (1958).
M. Frydman, G. Nilsson, T. Rengemo, and L. G. Sillén,Acta Chem. Scand. 12, 878 (1958).
F. J. Millero and V. Thurmond,J. Solution Chem. 12, 401 (1983).
K. S. Pitzer, J. Olsen, J. M. Simonsen, R. N. Roy, J. J. Gibbons, and L. Rowe,J. Chem. Eng. Data 30, 14 (1985).
E. J. Reardon and D. Langmuir,Am. J. Sci. 274, 599 (1974).
R. M. Pytkowicz and J. E. Hawley,Limnol. Oceanogr 19, 223 (1974).
P. J. Davies and R. Bubela,Chem. Geol. 12, 289 (1973).
R. A. Robie, O. Hemingway, and J. R. Fisher,U. S. Geol. Survey Bull. 1452, (1978).
W. D. Kline,J. Am. Chem. Soc. 51, 2093 (1929).
R. Marc and A. Šimek,Z. Anorg. Chem. 82, 17 (1913).
H. Gamsjäger,Thermodynamic Aspects of Dissolution Reactions in the System Mg 2+−Ca2+−CO2−H2 O. inMagnesite; Monograph Series on Mineral Deposits, Vol. 28, P. Möller, ed., (Gebrüder Bornträger, Berlin, 1989), pages 269–285.
J. A. Kittrick and F. J. Peryea,Soil Sci. Soc. Am. J. 50, 243 (1986).
R. Jantsch and F. Zemek,Radex-Rundschau 1965, 110 (1965).
P. W. Schindler, M. Reinert, and H. Gamsjäger,Helv. Chim. Acta 52, 2327 (1969).
W. F. Linke and A. SeidellSolubilities. Inorganic and Metal-Organic Compounds, Vol. 2 (American Chemical Society, Washington, 1965).
C. Milton and H. P. Eugster,Mineral Assemblages of the Green River Formation. In P. H. Abelson, ed.,Researches in Geochemistry, pages 118–150 (Wiley, New York, 1959).
K. S. Pitzer and G. Mayorga,J. Phys. Chem. 77, 2300 (1973).
J. C. Peiper and K. S. Pitzer,J. Chem. Thermodynamics 14, 613 (1982).
K. S. Pitzer and J. J. Kim,J. Am. Chem. Soc. 96, 5701 (1974).
R. N. Roy, J. J. Gibbons, D. P. Bliss, Jr., R. G. Casebolt and B. K. Baker,J. Solution Chem. 9, 911 (1980).
K. S. Pitzer,J. Solution Chem. 4, 249 (1975).
C. Monnin and J. Schott,Geochim. Cosmochim. Acta. 48, 571 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Königsberger, E., Schmidt, P. & Gamsjäger, H. Solid-solute phase equilibria in aqueous solution. VI. Solubilities, complex formation, and ion-interaction parameters for the system Na+−Mg2+−ClO −4 −CO2−H2O at 25°C. J Solution Chem 21, 1195–1216 (1992). https://doi.org/10.1007/BF00667217
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00667217