Abstract
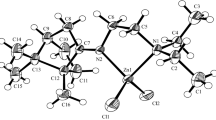
The X-ray crystal structure of (R,S)-α-amino-γ-caprolactone hydrochloride (compound1) and α-amino-γ-methyl-γ-valerolactone hydrochloride semihydrate (compound2) are presented. Both compound1 and compound2 belong to the orthorhombic system. Caprolactone-hydrochloride1 crystallizes in the space groupP212121 witha=5.1948(7),b=8.7404(8),c=17.907(1) Å.V=813.0(2) Å3,Z=4. Valerolactone-hydrochloride2 crystallizes in the space groupP na21 witha=26.771(8),b=5.1598(7),c=13.201(3) Å,V=1823.5(7) Å3,Z=8. The lactone cations maintain the same, open envelope conformation in both crystals. The lactone-hydrochloride packing arrangements in1 and2 are distinctly different. While in1 N−H...Cl and N−H...O hydrogen bonding creates two dimensional nets in the form of puckered layers perpendicular to the [001] direction, in2 a water molecule of crystallization with an additional OW−H...Cl hydrogen interaction assists in forming a three-dimensional hydrogen-bond network throughout the crystal.
Similar content being viewed by others
References
Armstrong, M.D.J. Am. Chem. Soc. 1949,71, 3399.
Deljac, A.; Kaitner, B.; Kirin, S.I.; Meštrović, E.Acta Crystallogr. Soc. C 1993,49, 1354.
Usher, J.J.; English, R.B.Acta Crystallogr. Sec. B 1978,34, 2012.
Bocelli, G.; Grenier-Loustalot, M.F.Acta Crystallogr. Soc. 1981,37, 2106.
Alcock, N.W.; Crout, D.H.G., Gregorio, M.V.M.; Lee, E.; Pike, G.; Samuel, C.J.;Phytochemistry 1989,21, 1835.
Papaioannou, D.; Barlos, K.; Francis, G.W.; Brekke, T.; Aksnes, D.W.; Marmann-Moe, K.Acta Chem. Scand. 1990,44, 189.
Kaitner, B.; Kirin, S.I.; Meštrović, E.Struct. Chem. 1994. Submitted for publication.
Fillman, J.; Albertson, N.J. Am. Chem. Soc. 1948,70, 171.
Goering, H.L.; Cristol, S.J.; Dittmer, K.J. Am. Chem. Soc. 1948,70, 3310.
Kirin, S.I.; Vela, V.; Deljac, A.Book of abstracts: 13th meeting of Croatian chemists; Zagreb: Croatia, 1993; p. 120.
Stoe & CieDIF4;Diffractometer control program; Version 7.0. Stoe & Cie, Darmstadt Germany; 1992.
Stoe & CieREDU4;Data Reduction Program; Version 7.0. Stoe & Cie. Darmstadt: Germany; 1992.
Walker, N.; Stuart, D.Acta Crystallogr. Sec. A 1983,39, 158.
Gabe, E.J.; Le Page, Y.; Charland, J.-P.; Lee, F.L.; White, P.S.J. Appl. Crystallogr. 1989,22, 384.
Rogers, D.Acta Crystallogr. Sec. A 1981,37, 734.
International Tables for X-ray Crystallography; Ibers, J.A.; Hamilton, W.C., Eds.; Kynoch Press: Birmingham, UK, 1974; Vol. IV, pp. 99–102, pp. 149–150. [Now distributed by Kluwer Academic Publishers, Dordrecht, The Netherlands.]
Johnson, C.K.ORTEPII. Report ORNL-5138. Oak Ridge National Laboratory: TN, 1976.
Nardelli, M.Comput. Chem. 1983,7, 95.
Steiner, T.; Saenger, W.J. Am. Chem. Soc. 1993,115, 4540.
Allen, F. H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.J. Chem. Soc. Perkin Trans II,1987, S1.
Bruvo, M.; Sikirica, M.; Vicković, I.Acta Crystallogr. Sec. B 1981,37, 700.
Matijašić, I.; Bocelli, G.; Ugozzoli, F.; Sgarabotto, P.Acta Crystallogr. Sec. C 1988,44, 159.
Matijašić, I.; Ugozzoli, F.; Bocelli, G.; Andretti, G.D.Acta Crystallogr. Sec. C 1989,45, 1546.
Mo, F.; Sivertsen, B.K.Acta Crystallogr. Sec. B 1971,27, 115.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kaitner, B., Kirin, S.I. & Meštrović, E. Two similar lactone-hydrochlorides with different types of hydrogen bonding networks: Crystal structure of (R,S)-α-amino-γ-caprolactone hydrochloride and racemic α-amino-γ-methyl-γ-valerolactone hydrochloride semihydrate. J Chem Crystallogr 25, 117–122 (1995). https://doi.org/10.1007/BF01665986
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01665986