Abstract
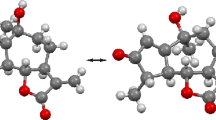
A new naturally occurring sesquiterpene lactone, 3β-acetoxy-8α, 9β-dihydroxy-1αH, 5αH, 6βH, 7αH-guaian-4(15), 10(14), 11(13)-trien-6,12-olide (Scheme 1) has been structurally characterized by X-ray diffraction methods. The compound crystallizes in the monoclinic space groupP21 with two molecules in the unit cell of dimensionsa=8.485(2),b=7.398(1),c=12.919(3) Å,β=98.17(3)°. The structure has been refined to a final value ofR of 0.039 for 2091 observed reflections. The molecule possesses a polyunsaturated guaianolide system which contains a twist chair exomethylenecycloheptane moiety,cis-fused with the exomethylene-cyclopentane [at C(1) and C(5)] andtrans-annelated with theα-exomethylene-γ-lactone at C(6) and C(7). The acetoxy substituent at C(3) isβ-oriented andtrans to the C(8) hydroxyl which, in turn, istrans to the neighboring C(9) hydroxyl. Substitution at C(9) is unprecedented among guaianolides isolated fromCentaureinae subtribe.
Similar content being viewed by others
References
Bovill, M. J., Guy, M. H. P., Sim, G. A., White, D. N. J., and Herz, W. (1979)J.C.S. Perkin 2, 53.
Daniewski, W. M., Nowak, G., Routsi, E., Rychlewska, U., Szczepańska, B., and Skibicki, P. (1992)Phytochemistry, submitted for publication.
Fischer, N. H., Olivier, E. J., and Fischer, H. D. (1979) InProgress in the Chemistry of Organic Natural Products, Vol. 38, W. Herz, H. Griesebach, and G. W. Kirby (eds.) (Springer Verlag, Vienna/New York).
Fronczek, F. R., Vargas, D., Parodi, F. J., and Fischer, N. H. (1989)Acta Crystallogr. C 45, 1829.
Johnson, C. K. (1965)ORTEP. Report ORNL-3794. (Oak Ridge National Laboratory, Tenn.).
Motherwell, W. D. S., and Clegg, W. (1978)PLUTO78. Program for plotting molecular and crystal structures. (Univ. of Cambridge, England).
Nardelli, M. (1983)Comput. Chem. 7, 95.
Rizzoli, C., Sangermano, V., Calestani, G., and Andreetti, G. D. (1986)CRYSRULER Package ver. 1.0. (Univ. of Parma, Italy).
Rychlewska, U. (1985)Acta Crystallogr. C 41, 540.
Rychlewska, U., and Kisiel, W. (1991)Acta Cryst. C 47, 129.
Sheldrick, G. M. (1986)SHELXS 86, Program for crystal structure solution. University of Göttingen, Federal Republic of Germany.
Sheldrick, G. M. (1976)SHELX 76, Program for crystal structure determination. (University of Cambridge, England).
Stevens, K. L., and Wong, R. Y. (1982)Cryst. Struct. Commun. 11, 949.
Thiessen, W. E., and Hope, H. (1970).Acta Crystallogr. B 26, 554.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rychlewska, U., Szczepańska, B., Daniewski, W.M. et al. Crystal structure of salograviolide A, a guaiane-type sesquiterpene lactone. Journal of Crystallographic and Spectroscopic Research 22, 659–663 (1992). https://doi.org/10.1007/BF01160982
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01160982