Abstract
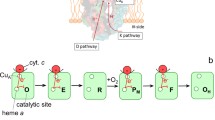
Warburg showed in 1929 that the photochemical action spectrum for CO dissociation from cytochrome c oxidase is that of a heme protein. Keilin had shown that cytochrome a does not react with oxygen, so he did not accept Warburg's view until 1939, when he discovered cytochrome a 3. The dinuclear cytochrome a 3-CuB unit was found by EPR in 1967, whereas the dinuclear nature of the CuA site was not universally accepted until oxidase crystal structures were published in 1995. There are negative redox interactions between cytochrome a and the other redox sites in the oxidase, so that the reduction potential of a particular site depends on the redox states of the other sites. Calculated electron-tunneling pathways for internal electron transfer in the oxidase indicate that the coupling-limited rates are 9×105 (Cu A → a) and 7×106 s−1 (a → a 3); these calculations are in reasonable agreement with experimental rates, after corrections are made for driving force and reorganization energy. The best CuA-a pathway starts from the ligand His204 and not from the bridging sulfur of Cys196, and an efficient a-a 3 path involves the heme ligands His378 and His376 as well as the intervening Phe377 residue. All direct paths from CuA to a 3 are poor, indicating that direct CuA → a 3 electron transfer is much slower than the CuA → a reaction. The pathways model suggests a means for gating the electron flow in redox-linked proton pumps.
Similar content being viewed by others
REFERENCES
Aasa, R., Albracht, S. P. J., Falk, K.-E., Lanne, B., and Vänngård, T. (1976). Biochim. Biophys. Acta 422, 260-272.
Ädelroth, P., Brzezinski, P., and Malmström, B. G. (1995). Biochemistry 34, 2844-2849.
Beinert, H. (1966). In The Biochemistry of Copper (Peisach, J., Aisen, P., and Blumberg, W. E., eds.), Academic Press, New York, pp. 213-234.
Beratan, D. N., Betts, J. N., and Onuchic, J. N. (1992). J. Phys. Chem. 96, 2852-2855.
Bertini, I., Bren, K. L., Clemente, A., Fee, J. A., Gray, H. B., Luchinat, C., Malmström, B. G., Richards, J. H., Sanders, D., and Slutter, C. E. (1996). J. Am. Chem. Soc. 118, 11658-11659.
Blair, D. F., Ellis, W. R., Jr., Wang, H., Gray, H. B., and Chan, S. I. (1986). J. Biol. Chem. 261, 11524-11537.
Boelens, R., Wever, R., and van Gelder, B. F. (1982). Biochim. Biophys. Acta 682, 264-272.
Brzezinski, P. (1996). Biochemistry 35, 5611-5615.
Brzezinski, P., Sundahl, M., Ädelroth, P., Wilson, M. T., El-Agez, B., Wittung, P., and Malmström, B. G. (1995). Biophys. Chem. 54, 191-197.
DeVault, D. (1971). Biochim. Biophys. Acta 225, 193-199.
Gray, H. B., and Winkler, J. R. (1996). Annu. Rev. Biochem. 65, 537-561.
Iwata, S., Ostermeier, C., Ludwig, B., and Michel, H. (1995). Nature 376, 660-669.
Karpefors, M., Slutter, C. E., Fee, J. A., Aasa, R., Källebring, B., Larsson, S., and Vänngård, T. (1996). Biophys. J. 71, 2823-2829.
Keilin, D., and Hartree, E. F. (1938). Nature 141, 870-871.
Keilin, D., and Hartree, E. F. (1939). Proc. Roy. Soc. London B127, 169-191.
King, T. E., Mason, H. S., and Morrison, M., eds. (1965). Oxidases and Related Redox Systems, Wiley, New York, pp. 587-588.
Lappalainen, P., Aasa, R., Malmström, B. G., and Saraste, M. (1993). J. Biol. Chem. 268, 26416-26421.
Malmström, B. G. (1973). Quart. Rev. Biophys. 6, 389-431.
Moser, C. C., Page, C. C., Chen, X., and Dutton, P. L. (1996). J. Bioenerg. Biomembr. 27, 263-274.
Moser, C. C., Page, C. C., Chen, X., and Dutton, P. L. (1997). J. Biol. Inorg. Chem. 2, 393-398.
Ramirez, B. E., Malmström, B. G., Winkler, J. R., and Gray, H. B. (1995). Proc. Natl. Acad. Sci. USA 92, 11949-11951.
Regan, J. J., and Onuchic, J. N. (1998) Adv. Chem. Phys., in press.
Sands, R. H., and Beinert, H. (1959). Biochem. Biophys. Res. Commun. 1, 175-179.
Slutter, C. E., Sanders, D., Wittung, P., Malmström, B. G., Aasa, R., Richards, J. H., Gray, H. B., and Fee, J. A. (1996). Biochemistry 35, 3387-3395.
Tsukihara, T., Aoyama, H., Yamashita, E., Tomizaki, T., Yamaguchi, H., Shinzawa-Ito, K., Nakashima, R., Yaono, R., and Yoshikawa, S. (1995). Science 269, 1069-1074.
van Gelder, B. F., and Beinert, H. (1969). Biochim. Biophys. Acta 189, 1-24
van Gelder, B. F., Orme-Johnson, W. H., Hansen, R. E., and Beinert, H. (1967). Proc. Natl. Acad. Sci. USA 58, 1073-1077.
Warburg, O., and Negelein, E. (1929). Biochem. Z. 214, 64-100.
Wikström, M., Krab, K., and Saraste, M. (1981). Cytochrome Oxidase--A Synthesis, Academic Press, London.
Williams, K. R., Gamelin, D. R., LaCroix, L. B., Houser, R. P., Tolman, W. B., Mulder, T. C., de Vries, S., Hedman, B., Hodgson, K. O., and Solomon, E. I. (1997). J. Am. Chem. Soc. 119, 613-614.
Wilmanns, M., Lappalainen, P., Kelly, M., Sauer-Eriksson, E., and Saraste, M. (1995). Proc. Natl. Acad. Sci. USA 92, 11955-11959.
Winkler, J. R., and Gray, H. B. (1997). J. Biol. Inorg. Chem. 2, 399-404.
Winkler, J. R., Malmström, B. G., and Gray, H. B. (1995). Biophys. Chem. 54, 199-209.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Regan, J.J., Ramirez, B.E., Winkler, J.R. et al. Pathways for Electron Tunneling in Cytochrome c Oxidase. J Bioenerg Biomembr 30, 35–39 (1998). https://doi.org/10.1023/A:1020551326307
Issue Date:
DOI: https://doi.org/10.1023/A:1020551326307