Abstract
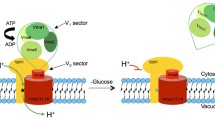
The filamentous fungusNeurospora crassa has many small vacuoles which, like mammalian lysosomes, contain hydrolytic enzymes. They also store large amounts of phosphate and basic amino acids. To generate an acidic interior and to drive the transport of small molecules, the vacuolar membranes are densely studded with a proton-pumping ATPase. The vacuolar ATPase is a large enzyme, composed of 8–10 subunits. These subunits are arranged into two sectors, a complex of peripheral subunits called V1 and an integral membrane complex called V0. Genes encoding three of the subunits have been isolated.vma-1 andvma-2 encode polypeptides homologous to the α and β subunits of F-type ATPases. These subunits appear to contain the sites of ATP binding and hydrolysis.vma-3 encodes a highly hydrophobic polypeptide homologous to the proteolipid subunit of vacuolar ATPases from other organisms. This subunit may form part of the proton-containing pathway through the membrane. We have examined the structures of the genes and attempted to inactivate them.
Similar content being viewed by others
References
Adachi, I., Arai, H., Pimental, R., and Forgac, M. (1990).J. Biol. Chem. 265, 960–966.
Ammerer, G., Hunter, C. P., Rothman, J. H., Saari, G. C., Valls, L. A., and Stevens, T. H. (1986).Mol. Cell. Biol. 6, 2490–2499.
Arai, H., Terres, G., Pink, S., and Forgac, M. (1988).J. Biol. Chem. 263, 8796–8892.
Arai, H., Pink, S., and Forgac, M. (1989).Biochemistry 28, 3075–3082.
Banta, L. M., Robinson, J. S., Klionsky, D. J., and Emr, S. D. (1988).J. Cell Biol. 107, 1369–1383.
Bowman, B. J., and Bowman, E. J. (1986).J. Membr. Biol. 94, 83–97.
Bowman, B. J., and Davis, R. H. (1977a).J. Bacteriol. 130, 274–284.
Bowman, B. J., and Davis, R. H. (1977b).J. Bacteriol. 130, 285–291.
Bowman, B. J., and Slayman, C. W. (1977).J. Biol. Chem. 252, 3357–3363.
Bowman, B. J., Allen, R., Wechser, M. A., and Bowman, E. J. (1988).J. Biol. Chem. 263, 14002–14007.
Bowman, B. J., Dschida, W. J., Harris, T., and Bowman, E. J. (1989).J. Biol. Chem. 264, 15606–15612.
Bowman, E. J. (1983).J. Biol. Chem. 258, 15238–15244.
Bowman, E. J., and Bowman, B. J. (1982).J. Bacteriol. 151, 1326–1337.
Bowman, E. J., and Bowman, B. J. (1988).Methods Enzymol. 157, 562–573.
Bowman, E. J., and Knock, T. E. (1992).Gene 114, 157–163.
Bowman, E. J., Mandala, S., Taiz, L., and Bowman, B. J. (1986).Proc. Natl. Acad. Sci. USA 83, 48–52.
Bowman, E. J., Tenney, K., and Bowman, B. J. (1988a).J. Biol. Chem. 263, 13994–14001.
Bowman, E. J., Siebers, A., and Altendorf, K. (1988b).Proc. Natl. Acad. Sci. USA 85, 7972–7976.
Cornelius, G., and Nakashima, H. (1987).J. Gen. Microbiol. 133, 2341–2347.
Cramer, C. L., Vaughn, L. E., and Davis, R. H. (1980).J. Bacteriol. 142, 945–952.
Cramer, C. L., Ristow, J. L., Paulus, T. J., and Davis, R. H. (1983).Anal. Biochem. 128, 384–392.
Denda, K., Konishi, J., Oshima, T., Date, T., and Yoshida, M. (1988).J. Biol. Chem. 263, 6012–6015.
Forgac, M. (1989).Physiol. Rev. 69, 765–795.
Foury, F. (1990).J. Biol. Chem. 265, 18554–18560.
Futai, M., Noumi, T., and Maeda, M. (1989).Annu. Rev. Biochem. 58, 111–136.
Gogarten, J. P., Kibak, H., Dittrich, P., Taiz, L., Bowman, E. J., Bowman, B. J., Manolson, M. F., Poole, R. J., Date, T., Oshima, T., Konishi, J., Denda, K., and Yoshida, M. (1989).Proc. Natl. Acad. Sci. USA 86, 6661–6665.
Gresser, M. J., Myers, J. A., and Boyer, P. D. (1982).J. Biol. Chem. 257, 12030–12038.
Grubmeyer, C., and Penefsky, H. S. (1981).J. Biol. Chem. 256, 3718–3727.
Gurr, S. J., Unkles, S. E., and Kinghorn, J. R. (1987). InGene Structure in Eukaryotic Microbes (Kinghorn, J. R., ed.), IRL Press, Oxford, pp. 93–139.
Hirsch, S., Strauss, A., Masood, K., Lee, S., Sukhatme, V., and Gluck, S. (1988).Proc. Natl. Acad. Sci. USA 85, 3004–3008.
Inatomi, K.-I., Eya, S., Maeda, M., and Futai, M. (1989).J. Biol. Chem. 264, 10954–10959.
Kasho, V. N., and Boyer, P. D. (1989).Proc. Natl. Acad. Sci. USA 86, 8708–8711.
Klionsky, D. J., Herman, P. K., and Emr, S. D. (1990).Microbiol. Rev. 54, 266–292.
Legerton, T. L., Kanamori, K., Weiss, R. L., and Roberts, J. D. (1983).Biochemistry 22, 899–903.
Mainzer, S. E., and Slayman, C. W. (1978).J. Bacteriol. 133, 584–592.
Mandel, M., Moriyama, Y., Hulmes, J. D., Pan, Y.-C. E., Nelson, H., and Nelson, N. (1988).Proc. Natl. Acad. Sci. USA 85, 5521–5524.
Manolson, M. F., Ouellette, B. F. F., Filion, M., and Poole, R. J. (1988).J. Biol. Chem. 263, 17987–17994.
Nelson, H., and Nelson, N. (1989).FEBS Lett. 247, 147–153.
Nelson, H., and Nelson, N. (1990).Proc. Natl. Acad. Sci. USA 87, 3503–3507.
Njus, D., Knoth, J., and Zallakian, M. (1981).Curr. Top. Bioenerg. 11, 107–147.
Ohya, Y., Umemoto, N., Tanida, I., Ohta, A., Iida, H., and Anraku, A. (1991).J. Biol. Chem. 266, 13971–13977.
Orbach, M. J., Porro, E. B., and Yanofsky, C. (1986).Mol. Cell. Biol. 6, 2452–2461.
Perin, M. S., Fried, V. A., Stone, D. K., Xie, X.-S., and Sudhof, T. C. (1991).J. Biol. Chem. 266, 3877–3881.
Rea, P. A., Griffith, C. J., Manolson, M. F., and Sanders, D. (1987).Biochim. Biophys. Acta 904, 1–12.
Sebald, W., and Wild, G. (1979).Methods Enzymol. 55, 344–351.
Selker, E. U., Cambareri, E. B., Jensen, B. C., and Haack, K. R. (1987).Cell 51, 741–752.
Senior, A. E. (1988).Physiol. Rev. 68, 177–231.
Sista, H. (1991). Ph.D. Thesis, University of California, Santa Cruz, California.
Umemoto, N., Yoshihisa, T., Hirata, R., and Anraku, Y. (1990).J. Biol. Chem. 265, 18447–18453.
Vaughn, L. E., and Davis, R. H. (1981).Mol. Cell. Biol. 1, 797–806.
Vogel, P. D., and Cross, R. L. (1991).J. Biol. Chem. 266, 6101–6105.
Walker, J. E., Saraste, M., Runswick, M. J., and Gay, N. J. (1982).EMBO J. 1, 945–951.
Yoshimori, T., Yamamoto, A., Moriyama, Y., Futai, M., and Tashiro, Y. (1991).J. Biol. Chem. 266, 17707–17712.
Zerez, C. R., Weiss, R. L., Franklin, C., and Bowman, B. J. (1986).J. Biol. Chem. 261, 8877–8882.
Zimniak, L., Dittrich, P., Gogarten, J. P., Kibak, H., and Taiz, L. (1988).J. Biol. Chem. 263, 9102–9112.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bowman, B.J., Vázquez-Laslop, N. & Bowman, E.J. The vacuolar ATPase ofNeurospora crassa . J Bioenerg Biomembr 24, 361–370 (1992). https://doi.org/10.1007/BF00762529
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00762529