Abstract
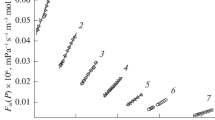
New experimental data on the viscosity of 12 organic liquids are presented at temperatures of 25, 30, 50, and 75°C and at pressures up to 110 MPa. The liquids measured are five n-alkanes (C6, C7, C8, C10, C12), cyclohexane, and six aromatic hydrocarbons (benzene, toluene, ethylbenzene, o-, m-, p-xylenes). The measurements were performed using a torsionally vibrating crystal method on a relative basis with an uncertainty less than 2%. A linear relationship between fluidity and molar volume, which is predicted from the hard-sphere theory, fails at pressures above 50 MPa. The rough hard-sphere model proposed by Chandler provides a reasonable representation of the data for aromatic hydrocarbons, while for n-alkanes the agreement is not satisfactory because of an aspherical shape of molecules. The viscosity data can be correlated well with the molar volume by a free-volume expression and also can be represented as a function of pressure by a similar expression to the Tait equation.
Similar content being viewed by others
References
B. Welber and S. L. Quimby, Phys. Rev. 107:645 (1957).
B. Welber, Phys. Rev. 119:1816 (1960).
J. K. Appeldoorn, E. H. Okrent, and W. Philippoff, Proc. Am. Petrol. Inst. III 42:163 (1962).
R. G. Rein, Jr., T. T. Charng, C. M. Sliepcevich, and W. J. Ewbank, ASLE Trans. 18:123 (1975).
A. F. Collings and E. McLaughlin, Trans. Faraday Soc. 67:340 (1971).
B. A. Lowry, S. A. Rice, and P. Gray, J. Chem. Phys. 40:3673 (1964).
D. E. Diller, J. Chem. Phys. 42:2089 (1965).
W. Herreman, A. Lattenist, W. Grevendonk, and A. DeBock, Physica 52:489 (1971).
R. W. H. Webeler and G. Allen, Phys. Rev. A 5:1820 (1972).
W. M. Haynes, Physica 67:440 (1973).
M. P. Bertinat, D. S. Betts, D. F. Brewer, and G. J. Butterworth, J. Low Temp. Phys. 16:479 (1974).
H. J. Strumpf, A. F. Collings, C. J. Pings, J. Chem. Phys. 60:3109 (1974).
D. E. Diller and J. M. Saber, Physica 108A:143 (1981).
H. J. M. Hanley, R. D. McCarty, and W. M. Haynes, J. Phys. Chem. Ref. Data 3:979 (1974).
J. H. Dymond and K. J. Young, Int. J. Thermophys. 1:331 (1980).
Y. Tanaka, unpublished data (obtained by correlation).
Selected Values in Physical and Thermodynamic Properties of Hydrocarbons and Related Compounds, API Research Project 44 (1953).
H. E. Eduljee, D. M. Newitt, and K. E. Weale, J. Chem. Soc. 3086 (1951).
J. W. M. Boelhouwer, Physica 26:1021 (1960).
P. S. Snyder, M. S. Benson, H. S. Huang, and J. Winnick, J. Chem. Eng. Data 19:157 (1974).
H. Kashiwagi, T. Fukunaga, Y. Tanaka, H. Kubota, and T. Makita, Rev. Phys. Chem. Japan 49:70 (1979).
Unpublished data obtained in the authors' laboratory.
A. M. Mamedov, T. S. Akhundov, and F. G. Abdullaev, Inzh.-fiz. Zh. 30:705 (1976).
J. D. Isdale, J. H. Dymond, and T. A. Brawn, High Temp.-High Press. 11:571 (1979).
S. O. Guseinov, Ya. M. Naziev, and A. K. Akhmedov, Izv. vyssh. ucheb. Zaved., Neft' i Gaz 16(2):65 (1973).
P. W. Bridgman, Proc. Am. Acad. Arts Sci. 61:57 (1926).
J. H. Dymond, K. J. Young, and J. D. Isdale, Int. J. Thermophys. 1:345 (1980).
J. H. Dymond, J. Robertson, and J. D. Isdale, Int. J. Thermophys. 2:133 (1981).
J. H. Dymond, J. Robertson, and J. D. Isdale, Int. J. Thermophys. 2:223 (1981).
N. A. Agaev and I. F. Golubev, Dokl. Akad. Nauk. SSSR 151:597 (1963).
N. A. Agaev and I. F. Golubev, Gazov. Promst. 8(7):50 (1963).
E. Dickinson, J. Phys. Chem. 81:2108 (1977).
D. W. Braizier and G. R. Freeman, Can. J. Chem. 47:893 (1969).
A. Jobling and A. S. C. Lawrence, Proc. Roy. Soc. London 206A:257 (1951).
H. J. Parkhurst, Jr., and J. Jonas, J. Chem. Phys. 63:2705 (1975).
A. M. Mamedov, T. S. Akhundov, Sh. M. Ismail-Zade, and A. D. Tairov, Izv. vyssh. ucheb. Zaved., Neft' i Gaz 14(2):74 (1971).
T. S. Akhundov, Sh. M. Ismail-Zade, and A. D. Tairov, Izv. vyssh. ucheb. Zaved., Neft' i Gaz 13(2):79 (1970).
T. S. Akhundov, Izv. vyssh. ucheb. Zaved., Neft' i Gaz 16(10):46, 74 (1973).
J. H. Dymond, J. Chem. Phys. 60:969 (1974).
D. Chandler, J. Chem. Phys. 62:1358 (1975).
J. H. Dymond and T. A. Brawn, in Proc. 7th Symp. Thermophys. Properties (ASME, New York, 1977), p. 660.
J. Jonas, D. Hasha, and S. G. Huang, J. Chem. Phys. 71:3996 (1979).
J. Jonas, D. Hasha, and S. G. Huang, J. Phys. Chem. 84:109 (1980).
B. J. Alder, D. M. Gass, and T. E. Wainwright, J. Chem. Phys. 53:3813 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kashiwagi, H., Makita, T. Viscosity of twelve hydrocarbon liquids in the temperature range 298–348 K at pressures up to 110 MPa. Int J Thermophys 3, 289–305 (1982). https://doi.org/10.1007/BF00502346
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00502346