Abstract
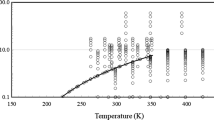
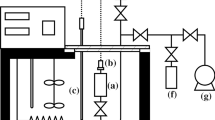
Studies of the thermodynamic properties of trifluoroiodomethane (CF3I) are presented in this paper. The vapor–liquid coexistence curve of CF3I was measured by visual observation of the meniscus. The critical temperature and the critical density of CF3I were determined by considering not only the level where the meniscus disappeared but also the intensity of the critical opalescence. The correlation of the saturated density in the critical region was developed, and the exponent of the power law β was determined. Correlations of the saturated vapor and liquid densities and the enthalpy of vaporization for CF3I were also developed. The vapor pressure of CF3I was measured at temperatures ranging from below the normal boiling point to the critical point, and a vapor pressure equation for CF3I was developed, from which the normal boiling point of CF3I was determined. The gaseous PVT properties of CF3I were measured with a Burnett/isochoric method, and a gaseous equation of state for CF3I was developed. The speed of sound of gaseous CF3I was measured with a cylindrical, variable-path acoustic interferometer operating at 156.252 kHz, and the ideal-gas heat capacity and second acoustic virial coefficient were calculated. A correlation of the second virial coefficient for CF3I was obtained by a semiempirical method using the square-well potential for the intermolecular force and was compared with the result based on PVT measurements. The surface tension of CF3I was measured with a differential capillary rise method (DCRM), and the temperature dependence of the results was successfully represented to within ±0.13 mN·m−1 using a van der Waals correlation.
Similar content being viewed by others
REFERENCES
L. Lankford and J. Nimitz, in Proc. International CFC and Halon Alternatives Conf. (Washington, D.C., 1993), p. 141.
M. S. Zhu, X. Y. Zhao, L. Shi, and L. Z. Han, J. Tsinghua Univ. 37:81 (1997).
Y. Y. Duan, L. Shi, M. S. Zhu, and L. Z. Han, J. Chem. Eng. Data 44:501 (1999).
I. B. Sladkov and A. V. Bogacheva, Zh. Priklad. Khim. 64:2435 (1991).
UNEP, 1994 Report of the Refrigeration, Air Conditioning and Heat Pumps Technical Options Committee (Nairobi, UNEP, 1995).
Y. Y. Duan, M. S. Zhu, and L. Z. Han, Fluid Phase Equil. 121:227 (1996).
J. M. H. Levelt Sengers and J. V. Sengers, in Perspectives in Statistical Physics, H. J. Raveche, ed. (North-Holland, Amsterdam, 1981), Chap. 14.
H. J. Emeleus and J. F. Wood, J. Chem. Soc. 2153 (1948).
E. A. Nodiff, A. V. Grosse, and M. Hauptshein, J. Org. Chem. 235 (1953).
R. N. Haszeldine, J. Chem. Soc. 4259 (1952).
N. N. Iarovenko, J. Gen. Chem. USSR 28:2543 (1958).
B. J. Zwolinski, Thermodynamics Research Center Data Project, Thermodynamics Research Center, Loose-leaf data sheets (Texas A & M University, College Station, Texas, 1976).
Y. Y. Duan, M. S. Zhu, and L. Z. Han, Fluid Phase Equil. 131:233 (1997).
S. A. Kudchadker and A. P. Kudchadker, J. Phy. Chem. Ref. Data 7:425 (1978).
Y. Y. Duan, L. Q. Sun, L. Shi, M. S. Zhu, and L. Z. Han, Fluid Phase Equil. 137:121 (1997).
Y. Y. Duan, L. Shi, M. S. Zhu, and L. Z. Han. Fluid Phase Equil. 154:71 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Duan, Y.Y., Shi, L., Sun, L.Q. et al. Thermodynamic Properties of Trifluoroiodomethane (CF13I)1 . International Journal of Thermophysics 21, 393–404 (2000). https://doi.org/10.1023/A:1006683529436
Issue Date:
DOI: https://doi.org/10.1023/A:1006683529436