Summary
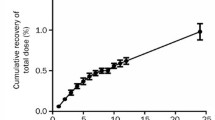
N-pentyl-sparsomycin (PSm) is a lipophilic analogue of sparsomycin (Sm), which is a well known inhibitor of protein synthesis. This compound was selected for preclinical pharmacokinetic studies because of its high in vitro and in vivo antitumor activity. In this study in which the drug was evaluated in beagle dogs under anaesthesia, the drug concentrations in plasma, urine and bile samples were determined using high performance liquid chromatography (HPLC). Plasma protein binding was approximately 54%. The mean t1/2 β was 0.2 hours (12 minutes) and t1/2 τ was 0.75 ± 0.1 hours (45 ± 6 minutes). During continuous infusions up to 5.25 hours, the steady state was reached in 3 out of 6 experiments, suggesting that in some cases the real t1/2 τ was longer than measured. PSm was actively reabsorbed from the renal tubuli. This process was saturable at the higher doses. Tubular reabsorption played only a minor role in pharmacokinetics as most of the drug (67%) was eliminated by the non-renal clearance. The non-renal clearance was saturable at higher doses of PSm and was the reason for non-linearity of pharmacokinetics.
Similar content being viewed by others
References
Owen SP, Dietz A, Camiener GW: Sparsomycin, a new antitumor antibiotic. I.; Discovery and biological properties. Antimicrob Agents 772–779, 1962
Monro RE, Celma ML, Vazquez D: Action or sparsomycin on ribosome-catalyzed peptidyl transfer. Nature 222:356–358, 1969
Clinical Brochure. Sparsomycin (NSC-59729), National Cancer Inst, 1964
Close HP, McFarlane JR: Ocular toxicity with sparsomycin (NSC-59729) in a phase I study: A preliminary report. Cancer Chemother Rep 43:29–31, 1964
Ottenheijm HCJ, Liskamp RMJ, Van Nispen SP, Boots HA, Tyhuis MW: Total synthesis of the antibiotic sparsomycin, a modified amino acid monoxodithioacetal. J Org Chem 46:3272–3283, 1981
Liskamp RMJ, Colstee JH, Ottenheijm HCJ, Lelieveld P, Akkerman W: Structure-activity relationships of sparsomycin and its analogues. Octylsparsomycin: the first analogue more active than sparsomycin. J Med Chem 27:301–306, 1984
Van Den Broek LAGM, Liskamp RMJ, Colstee JH, Lelieveld P, Remacha M, Vazquez D, Ballesta JPG, Ottenheijm HCJ: Structure-activity relationships of sparsomycin and its analogues. Inhibition of peptide bond formation in cell-free systems and L1210 and bacterial cell growth. J Med Chem 30:325–333, 1987
Zylicz Z, Wagener DJT, Van Rennes H, Van Der Kleijn E, Van Den Broek LAGM, Ottenheijm HCJ: In vivo antitumor activity of sparcomycin and its analogues in eight murine tumor models. Invest New Drugs 6:285–292, 1988
Zylicz Z, Wagener DJT, Garzotto M, Vree TB, Van Der Kleijn E, Ballesta JPB, Lelieveld P, Van Den Broek LAGM, Ottenheim HCJ: Comparison of in vitro and in vivo biological activity of two homologous series of sparsomycin analogues. In: Progress in Antimicrobial and Anticancer Therapy. Berkada B, Kuemmerle HP (eds), Munich 1987, pp 297–299.
Van Den Broek LAGM, Lazaro E, Zylicz Z, Fennis PJ, Missler FAN, Lelieveld P, Garzotto M, Wagener DJT, Ballesta JPG, Ottenheijm HCJ: Lipophilic analogues of sparsomycin as strong inhibitors of protein synthesis and tumor growth: a structure-activity relationship study. J Med Chem 32:2002–2015, 1989
Winograd B, Oosterbaan MJM, Van Der Kleijn E, Liskamp RMJ, Ottenheijm HCJ, Wagener DJT: Determination of sparsomycin in plasma and urine of the dog by means of reversed-phase high-performance liquid chromatography and first pharmacokinetic results. J Chromatogr (Biomed Appl) 275:145–153, 1983
Heckman P, Van Ginneken CAM: Kinetic modelling of the renal excretion of iodopyracet in the dog. J Pharmacokin Biopharm 10:77–92, 1982
Zylicz Z, Wagener DJT, Fernandez del Moral P, Vree TB, Van Der Kleijn E, Winograd B, Van Haelst U, Van Den Broek LAGM, Ottenheijm HCJ: Pharmacokinetics and toxicology of sparsomycin in beagle dogs. Cancer Chemother Pharmacol 20:115–124, 1987
Zylicz Z, Wagener DJT, Garzotto M, Vree TB, Van Der Kleijn E, Van Den Broek LAGM, Ottenheijm HCJ: Preclinical pharmacokinetics of the antibiotic deshydroxysparsomycin in beagle dogs. Anticancer Res 8:1381–1386, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zylicz, Z., Wagener, D.J.T., Garzotto, M. et al. Pharmacokinetics of the antitumor antibiotic n-pentyl-sparsomycin in beagle dogs. Invest New Drugs 8, 25–32 (1990). https://doi.org/10.1007/BF00216921
Issue Date:
DOI: https://doi.org/10.1007/BF00216921