Summary
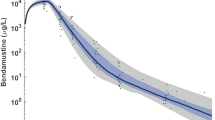
Mitoguazone is a unique chemotherapeutic agent whose activity is believed to result primarily from the competitive inhibition of S-adenosyl-methionine decarboxylase leading to a disruption in polyamine biosynthesis. Initial clinical trials demonstrated that the dose-limiting toxicities (mucositis and myelosuppression) of Mitoguazone were both dose and schedule dependent. Early pharmacokinetic studies of Mitoguazone in man revealed a prolonged half-life. Concurrent with a recent Phase II trial of Mitoguazone in patients with AIDS related non-Hodgkin's lymphoma, the single dose pharmacokinetics of Mitoguazone were characterized. Twelve patients received 600 mg/m2 of intravenous Mitoguazone over 30 minutes on an intermittent every 2 week schedule. Blood, urine, cerebrospinal fluid (CSF), pleural fluid and tissue samples were collected and analyzed by HPLC. Mitoguazone was cleared from the plasma triexponentially with a harmonic mean terminal half-life of 175 hours and a mean residence time of 192 hours. Peak plasma levels occurred immediately post-infusion, ranged from 6.47 to 42.8 μg/ml, and remained (for an extended period) well above the reported concentration for inhibition of polyamine biosynthesis. Plasma clearance averaged 4.73 l/hr/m2 with a relatively large apparent volume of distribution at steady-state of 1012 l/m2 indicating tissue sequestration. Renal excretion of unchanged Mitoguazone accounted for an average of 15.8% of the dose within 48 to 72 hours post-administration. Detectable levels of drug were present in random voided samples eight days post-dose. Mitoguazone levels in CSF ranged from 22 to 186 ng/ml post-dose with CSF/plasma ratios ranging from 0.6% to 7%. The pleural fluid/plasma ratio was approximately 1. Tissue levels of Mitoguazone were highest in the liver followed by lymph node, spleen and the brain.
Similar content being viewed by others
References
Warrell RP, Burchenal JH: Methyl-glyoxal-bis (guanylhydrazone) (methyl-GAG): current status and future prospects. J Clin Oncol 1:52–65, 1983
Lim SW, Look RM, Bick AB, Hamburg SI, Lawrence GN, Fuerst MP, Kusuanco DA, Kessler CE, Giles FJ: MGBG therapy of relapsed extralymphatic HIV-associated non-Hodgkin's lymphoma (HIV NHL). Proc Am Soc Clin Oncol 14:404, 1995
Von Hoff DD: MGBG: Teaching an old drug new tricks. Annals of Onc 5:487–493, 1994
Morris D, Jorstad C, Seyfried CE: Inhibition of the synthesis of polyamines and DNA in activated lymphocytes by a combination of methylornithine and methyl-glyoxal-bis (guanylhydrazone). Cancer Res 37:3169–3172, 1977
Burk D, Evans W, Hunter J, Woods M: Primary inhibition of respiration by methylglyoxal-bis (guanylhydrazone) in L1210 leukemia and other cells, with implications for multiple chemotherapy. Proc Am Assoc Cancer Res 3:308, 1962
Marsh K, Repta A, Sternson L: High-performance liquid Chromatographic analysis of the anticancer agent methylglyoxal bis (guanylhydrazone) (MGBG, NSC #32946) in biological fluids. J Chromat 187:101–109, 1980
Hart RD, Roboz J, Wu K, Bruckner H, Ohnuma T, Holland JF: Clinical and pharmacological studies with weekly and biweekly methyl-glyoxal bis-guanylhydrazone (methyl-G). Proc Am Assoc Cancer Res 21:181, 1980
Marsh KC, Liesmann J, Patton TF, Fabian CJ, Sternson LA: Plasma levels and urinary excretion of methyl-GAG following IV infusion in man. Cancer Treatment Rep 65:253–257, 1981
Rosenblum MG, Keating MJ, Yap BS, Loo TL: Pharmacokinetics of [14C]methylglyoxal-bis(guanylhydrazone) in patients with leukemia. Cancer Res 41:1748–1750, 1981
Oliverio VT, Adamson RH, Henderson ES, Davidson JD: The distribution, excretion and metabolism of methylglyoxal-bis-guanylhydrazone-C14. J Pharmacol Exp Ther 141:149–156, 1963
Stewart DJ, Rosenblum MG, Luna M, Loo TL: Disposition of methylglyoxal bis(guanylhydrazone) (MGBG, NSC-32946) in man. Cancer Chemother Pharmacol 7:31–35, 1981
Rosenblum MG, Stewart DJ, Yap BS, Leavens M, Benjamin RS, Loo TL: Penetration of methylglyoxal bis(guanylhydrazone) into intracerebral tumors in humans. Cancer Res 41:459–462, 1981
Hauswald C: In vitro blood cell partitioning and plasma protein binding of 14C-WIN 10708-2 in human blood. Sanofi Research Division, Study Report No. 2107, 30 March 1995 (Revised 18 May 1995)
Helaszek C: Investigation of human metabolism of WIN 10708-2 using human liver microsomes and precisioncut slices. Sanofi Research Division, Study Report No. 2176, 04 August 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rizzo, J., Levine, A.M., Weiss, G.R. et al. Pharmacokinetic profile of Mitoguazone (MGBG) in patients with AIDS related non-Hodgkin's lymphoma. Invest New Drugs 14, 227–234 (1996). https://doi.org/10.1007/BF00210796
Issue Date:
DOI: https://doi.org/10.1007/BF00210796