Abstract
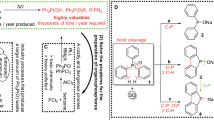
In water, metallic palladium on carbon was found to catalyze the deep oxidation of organophosphorus and organosulfur compounds by dioxygen at 90°C in the presence of carbon monoxide. This system presents the first examples of catalytic cleavage of phosphorus-carbon bonds. Starting with trimethylphosphine oxide, the phosphorus-containing products formed by sequential P-C cleavage were dimethylphosphinic acid, methylphosphonic acid, and phosphoric acid. A similar reaction sequence was also observed for triethylphosphine oxide, except that products formed by partial oxidation of the ethyl groups, such as phosphonoacetic acid, were also seen as intermediates. The deep oxidation of dimethyl and diethyl sulfides proceeded through the intermediacy of the corresponding sulfoxides. For the methyl derivatives, the ease of oxidation decreased in the order: (CH3)2S>(CH3)2S O>(CH3)2SO2 and is consistent with the system acting as an electrophilic oxidant.
Similar content being viewed by others
References
U.S. Chemical Weapons Stockpile Information, declassified News Release, Office of Assistant Secretary of Defense (Public Affairs),Washington, 22 January 1996.
L. Ember,Chem. Eng.News 74 (1996) 9.
National Research Council, Review and Evaluation of Alternative Chemical Disposal Technologies (National Academy Press, Washington, 1996).
National Research Council, Alternative Technologies for the Destruction of Chemical Agents and Munitions (National Academy Press, Washington, 1993).
K.J. Flamm, Q. Kwan and W.B. McNulty, Chemical Agent and Munitions Disposal: Summary of the U.S. Army's Experience, report SAEPEO-CDE-IS-87005 (1987).
S.M. Somani, ed., Chemical Warfare Agents (Academic Press, SanDiego, 1992).
P.E.Garrou, Chem.Rev. 85 (1985) 171, and references therein.
A.J. Carty, Pure Appl. Chem. 54 (1982) 113, and references therein.
A.Y. Aksinenko, A.N. Pushin and V.B. Sokolov, Phosphorus, Sulfur, and Silicon 84 (1993) 249.
M. Lin and A. Sen, J.Am.Chem. Soc. 114 (1992) 7307; A. Sen and M. Lin, US Patent 5,393,922 (1995).
L.W.Gosser, US Patent 4,681,751 (1987).
T. Hogan, R. Simpson, M. Lin and A. Sen, Catal. Lett. 40 (1996) 95.
Rights and permissions
About this article
Cite this article
Hogan, T., Simpson, R., Lin, M. et al. The deep oxidation of chemical warfare agent models: facile catalytic oxidative cleavage of phosphorus-carbon and sulfur-carbon bonds using dioxygen. Catalysis Letters 49, 59–63 (1997). https://doi.org/10.1023/A:1019088818029
Issue Date:
DOI: https://doi.org/10.1023/A:1019088818029