Abstract
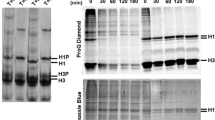
A variety of nonhistone proteins and polyamines has been studied for their substrate activity for nuclear histoneN-acetyltransferase. Nonhistone chromatin high-mobility group (HMG) proteins are found to be as good a substrate for the enzyme as histones. The enzyme also acetylates spermidine and spermine. However, protamine, bovine serum albumin, and ubiquitin are not substrates. Chymotryptic peptides of histone and HMGs retained about 64% of the substrate activity, but trypsin treatment reduced the substrate activity by more than 85%. BothN-acetyltransferase activities for HMGs and histones are copurified through salt extraction, polyethylene glycol fractionation, and chromatography on DEAE-cellulose, phosphocellulose columns, and a HPLC anionic-exchange column. The highly purified nuclear histone acetyltransferase shows similar optimalpH and ping-pong kinetics for both HMGs and histones. TheK m for HMG is 0.25 mg/ml. HMGs are able to accept the acetyl group from isolated acetyl-enzyme intermediate. Denatured gel analysis shows that HMG 1 and HMG 2 are the major proteins acetylated. High salt concentrations, mononucleotides, and DNA, which inhibit histone substrate activity of the enzyme, also inhibit HMG substrate activity. These observations suggest that there is a major nuclearN-acetyltransferase which is responsible for the acetylation of both histones and HMGs and perhaps also of spermine and spermidine. Thus the regulation of the structure and function of chromatin through postsynthetic acetylation can be achieved by a single nuclearN-acetyltransferase.
Similar content being viewed by others
References
Alberts, B. M., Worcel, A., and Weintraub, H. (1977). On the biological implications of chromatin structure. In Bradbury, E. M., and Javaherian, K. (eds.),The Organization and Expression of the Eukaryotic Genome Academic Press, New York, pp. 165–191.
Allegra, P., Sterner, R., Clayton, D. F., and Allfrey, V. G. (1987). Affinity chromatographic purification of nucleosomes containing transcriptionally active DNA sequences.J. Mol. Biol. 196379.
Belikoff, E., Wong, L.-J., and Alberts, B. M. (1980). Extensive purification of histone acetylase A, the major histone N-acetyltransferase activity detected in mammalian cell nuclei.J. Biol. Chem. 25511448.
Blankenship, J., and Walle, T. (1977). Acetylation of spermidine and spermine by rat liver and kidney chromatin.Arch. Biochem. Biophys. 179235.
Candido, E. P. M., and Dixon, G. H. (1972). Amino terminal sequences and sites ofin vivo acetylation of trout testis histones III and IIb2.Proc. Natl. Acad. Sci. USA 692015.
Cooper, E., and Spaulding, S. W. (1985). Hormonal control of the phosphorylation of histones, HMG proteins and other nuclear proteins.Mol. Cell Endocrinol. 391.
Csordas, A. (1990). On the biological role of histone acetylation.Biochem. J. 26523.
Cullis, P. M., Wolfenden, R., Cousens, L. S., and Alberts, B. (1982). Inhibition of histone acetylation by N-[2-(S-coenzyme A) acetyl] spermidine amide, a multisubstrate analog.J. Biol. Chem. 25712165.
Davie, J. R., and Candido, E. P. M. (1978). Acetylated histone H4 is preferentially associated with template-active chromatin.Proc. Natl. Acad. Sci. USA 753574.
deMurcia, G., Huletsky, A., and Poirier, G. G. (1988). Modulation of chromatin structure by poly(ADP-ribosyl)ation.Biochem. Cell Biol. 66625.
Elton, T. S., and Reeves, R. (1986). Purification and postsynthetic modification of Friend erythroleukemic cell high mobility group protein HMG-1.Anal. Biochem. 15753.
Erwin, G. G., Persson, L., and Pegg, A. E. (1984). Differential inhibition of histone and polyamine acetylases by multisubstrate analogues.Biochemistry 234250.
Gallwitz, D. (1970). Extraction and partial purification of two histone-specific transacetylases from rat liver nuclei.Biochem. Biophys. Res. Commun. 40236.
Gershey, E. L., Visali, G., and Allfrey, V. G. (1968). Chemical studies of histone acetylation. The occurrence of ε-N-acetyllysine in the f2a1 histone.J. Biol. Chem. 2435081.
Goldknopf, I. L., Rosenbaum, F., Sterner, R., Vidali, G., Allfrey, V. G., and Busch, H. (1979). Phosphorylation and acetylation of chromatin conjugate protein A24.Biochem. Biophys. Res. Commun. 90269.
Goodwin, G. H., Nicolas, R. H., and Johns, E. W. (1975). An improved large scale fractionation of high mobility group non-histone chromatin proteins.Biochim. Biophys. Acta 405280.
Goodwin, G. H., Walker, J. M., and Johns, E. W. (1978). Studies on degradation of high mobility group nonhistone chromosomal proteins.Biochim. Biophys. Acta 519233.
Hebbes, T., Thorne, A. W., and Crane-Robinson, C. (1988). A direct link between core histone acetylation and transcriptionally active chromatin.EMBO J. 71395.
Ip, Y. T., Jackson, V., Meier, J., and Chalkley, R. (1988). The separation of transcriptionally engaged genes.J. Biol. Chem. 26314044.
Kelner, D. N., and McCarty, K. S., Sr. (1984). Porcine liver nuclear histone acetyltransferase.J. Biol. Chem. 2593413.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature 227680.
Libby, P. R. (1978). Calf liver nuclear N-acetyltransferases: Purification and properties of two enzymes with both spermidine acetyltransferase and histone acetyltransferase activities.J. Biol. Chem. 253233.
Libby, P. R. (1980). Rat liver nuclear N-acetyltransferases: Separation of two enzymes with both histone and spermidine acetyltransferase activity.Arch. Biochem. Biophys. 203384.
McCarty, K. S., Sr., Kelner, D. N., Wilke, K., and McCarty, K. S., Jr. (1982). Role of HMG-nucleosome complexes in eukaryotic gene activity. In Padilla, G. M., and McCarty, K. S., Sr. (eds.),Genetic Expression in the Cell Cycle Academic Press, New York, pp. 55–102.
Pasqualini, J. R., Sterner, R., Mercat, P., and Allfrey, V. G. (1989). Estradiol enhanced acetylation of nuclear high mobility group proteins of the uterus of newborn guinea pigs.Biochem. Biophys. Res. Commun. 1611260.
Pegg, A. E., Wechter, R. S., Clark, R. S., Wiest, L., and Erwin, B. G. (1986). Acetylation of decarboxylated S-adenosylmethionine by mammalian cells.Biochemistry 25379.
Prasad, S., and Thakur, M. K. (1989).In vitro acetylation of the liver HMG non-histone proteins and its modulation by spermine and dexamethasone during aging of rats.Mol. Biol. Rep. 13221.
Sharpe, D. J., and Wong, L.-J. C. (1990). Effects of substrates on the thermal stability of nuclear histone acetyltransferase.Biochimie 72323.
Simpson, R. T. (1978). Structure of chromatin containing extensively acetylated H3 and H4.Cell 13691.
Sterner, R., Vidali, G., Heinrikson, R., and Allfrey, V. (1978). Postsynthetic modification of high mobility group proteins. Evidence that high mobility group proteins are acetylated.J. Biol. Chem. 2537601.
Sterner, R., Vidali, G., and Allfrey, V. (1979). Studies of acetylation and deacetylation in high mobility group proteins. Identification of the sites of acetylation in HMG-1.J. Biol. Chem. 25411577.
Sterner, R., Vidali, G., and Allfrey, V. (1981). Studies of acetylation and deacetylation in high mobility group proteins. Identification of the sites of acetylation in High Mobility Group proteins 14 and 17.J. Biol. Chem. 2568892.
Sures, I., and Gallwitz, D. (1980). Histone-specific acetyltransferases from calf thymus.Biochemistry 19943.
Vidali, G., Boffa, L. C., Bradbury, E. M., and Allfrey, V. G. (1978). Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histone H3 and H4 and increased DNase I sensitivity of the associated DNA sequences.Proc. Natl. Acad. Sci. USA 752239.
Wiktorowicz, J. E., and Bonner, J. (1982). Studies on histone acetyltransferase.J. Biol. Chem. 2572893.
Wiktorowicz, J. E., Campos, K. L., and Bonner, J. (1981). Substrate and product inhibition initial rate kinetics of histone acetyltransferase.Biochemistry 201464.
Wong, L.-J. C. (1980). Effect of sea urchin sperm chromatin on histone acetylation.Biochem. Biophys. Res. Commun. 971362.
Wong, L.-J. C., and Alberts, B. M. (1977). Studies on histone acetylation.Fed. Proc. 36748.
Wong, L.-J. C., and Patton, W. F. (1985). Salt inhibition of nuclear histone acetyltransferase from calf thymus.Int. J. Biochem. 17123.
Wong, L.-J. C., and Sharpe, D. J. (1991). Regulation of nuclear histone acetyltransferase by nucleic acids, histone DNA complex and chromatin.Biochem. Genet. 2913.
Wong, L.-J. C., and Wong, S. S. (1983). Kinetic mechanism of the reaction catalyzed by nuclear histone acetyltransferase from calf thymus.Biochemistry 224637.
Wu, R. S., Panusz, H. T., Hatch, C. L., and Bonner, W. M. (1986). Histones and their modifications.CRC Crit. Rev. Biochem. 20201.
Zhang, D. E., and Nelson, D. A. (1988). Histone acetylation in chicken erythrocytes: Rates of acetylation and evidence that histones in both active and potentially active chromatin are rapidly modified.Biochem J. 250857.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wong, LJ.C., Sharpe, D.J. & Wong, S.S. High-mobility group and other nonhistone substrates for nuclear histoneN-acetyltransferase. Biochem Genet 29, 461–475 (1991). https://doi.org/10.1007/BF02399688
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02399688