Abstract
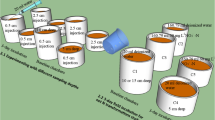
The effects of elevated atmospheric SO 2−4 deposition on S cycling in forest soils were assessed in an irrigation experiment using stable S isotopes. Over a period of 20 months, core lysimeters of five acidic forest soils from Southern Germany with different parent material and pedogenesis were irrigated with solutions chemically similar to canopy throughfall. Sulfate deposition in three experimental variants corresponded to 23, 42 and 87 kg S ha−1 yr−1. The SO 2−4 used for irrigation had aδ 34S ratio of +28.0‰ CDT (Canon Diablo Troilite standard), differing by more than +25‰ from natural and anthropogenic S in Southern Germany.
A combination of chemical and isotopic analyses of soil and seepage water samples was used to elucidate the fluxes and transformations of simulated wet SO 2−4 deposition in each soil core. Retention of experimentally deposited S ranged from 57±5% in coarse-grained soils low in sesquioxides and clay, to 80±8% in loamy soils with high sesquioxide content. The sesquioxide content proved to be the major factor governing S retention. The ratio of S retained as inorganic SO 2−4 (mainly by adsorption) to that incorporated into organic compounds (presumably by microbial synthesis) ranged from 2 to 4. For the organic S pool, the amount of S retained as C-bonded S exceeded by far that immobilized as ester sulfate in four of the five soils.
Application of34S-enriched SO 2−4 appears to be a suitable experimental tool to assess fluxes and transformations of deposited S in forest soils, if aerobic conditions are maintained. In contrast to radioactive S tracers, the concept should be applicable not only in laboratory and lysimeter experiments, but also in long term studies of whole forest ecosystems (e.g., experimental watersheds).
Similar content being viewed by others
References
Connell, W. E. and Patrick, W. H.: 1969, ‘Reduction of Sulphate to Sulfide in Waterlogged Soil,’Soil Sci. Soc. Am. Proc. 33, 711–715.
David, M. B., Mitchell, M. J. and Aldcorn, D.: 1989, ‘Analysis of Sulfur in Soil, Plant and Sediment Materials: Sample Handling and Use of an Automated Analyzer,’Soil Biol. Biochem. 21, 119–123.
Farmer, V. C., Russell, J. D. and Berrow, M. L.: 1980, ‘Imogolite and Proto-imogolite Allophane in Spodic Horizons: Evidence for a Mobile Aluminium Silicate Complex in Podzol Formation,’J. Soil Science 31, 673–684.
Fitzgerald, J. W., Ash, J. T., Strickland, T. C. and Swank, W. T.: 1983, ‘Formation of Organic Sulfur in Forest Soils — A Biologically Mediated Process,’Can. J., For. Res. 13, 1077–1082.
Giesemann, A. and JÄger, H. J.: 1993, ‘Untersuchungen zum Schwefelhaushalt von Waldökosystemen (Schwarzwald) mittels stabiler Schwefelisotope. II. RÄumliche VariabilitÄt der S-Isotopenverteilung,’ inProjekt EuropÄisches Forschungszentrum für MaΒnahmen zur Luftreinhaltung (PEF) im Kernforschungszentrum Karlsruhe, KfK-PEF-Berichte 104, pp. 55–65.
Gobran, G. R. and Nilsson, S. J.: 1988, ‘Effects of Forest Floor Leachate on Sulfate Retention in a Spodosol Soil,’J. Environ. Qual. 17, 235–239.
Inskeep, W. P.: 1989, ‘Adsorption of Sulfate by Kaolinite and Amorphous Iron Oxide in the Presence of Organic Ligands,’J. Environ. Qual. 18, 379–385.
Johnson, C. M. and Nishita, H.: 1952, ‘Microestimation of Sulfur in Plant Materials, Soils, and Irrigation Waters,’Anal. Chem. 24, 736–742.
Krouse, H. R. and Tabatabai, M. A.: 1986, ‘Stable Sulfur Isotopes’, in M. A. Tabatabai (ed.),Sulfur in Agriculture, American Society of Agronomy Monograph27, Madison, pp. 169–205.
Krouse, H. R., Stewart, J. W. B. and Grinenko, V. A.: 1991, ‘Pedosphere and Biosphere’, in H. R. Krouse, and V. A. Grinenko, (eds.)Stable Isotopes: Natural and Anthropogenic Sulphur in the Environment, Scope43, J. Wiley &; Sons, Chichester, pp. 267–306.
Mayer, B.: 1993,Untersuchungen zur Isotopengeochemie des Schwefels in Waldböden und neu gebildetem Grundwasser unter Wald, GSF-Bericht 2/93, GSF Forschungszentrum für Umwelt und Gesundheit, Institut für Hydrologie, Oberschleissheim, Germany.
McKeague, J. A., Brydon, J. E. and Miles, N. M.: 1971, ‘Differentiation of Forms of Extractable Iron and Alumium in Soils,’Soil Sci. Soc. Am. Proc. 35, 33–38.
Nordstrom, D. C.: 1982, The Effect of Sulfate on Aluminium Concentrations in Natural Waters: Stability Relations in the system Al2O3-SO3-H2O at 298 K’,Geochim. Cosmochim. Acta 46, 681–692.
Prietzel, J.: 1993, Auswirkung definierter Schwefelbelastung auf die chemischen Eigenschaften von Waldböden — Auswertung zweier Lysimeterversuche. Thesis, Faculty of Forestry, University of Munich, Germany.
Rajan, S. S. S.: 1978, ‘Sulfate Adsorbed on Hydrous Alumina, Ligands Displaced and Changes in Surface Charge,’Soil Sci. Soc. Am. J. 42, 39–44.
Reuss, J. O. and Johnson, D. W.: 1986,Acid Deposition and the Acidification of Soils and Waters, Ecological Studies59, Springer Verlag, New York.
Schindler, S. C., Mitchell, M. J., Scott, T. C., Fuller, R. D. and Driscoll, C. T.: 1986, ‘Incorporation of Sulfur-35 Sulfate into Inorganic and Organic Constitutents of Two Forest Soils,’Soil Sci. Soc. Am. J. 50, 457–462.
Schwertmann, U.: 1959, ‘Die fraktionierte Extraktion der freien Eisenoxyde im Boden, ihre mineralogischen Formen und ihre Entstehungsweisen’,Z. Pflanzenern. Bodenk. 84, 194–204.
Singh, B. R.: 1980, ‘Distribution of Total Extractable Sulfur and Adsorbed Sulfur-35 Labeled Sulfate in Some Acid Forest Soil Profiles of Southern Norway,’Acta Agric. Scand. 30, 357–363.
Strickland, T. C. and Fitzgerald, J. W.: 1984, ‘Formation and Mineralization of Organic Sulfur in Forest Soils,’Bio geochemistry 1, 79–95.
Strickland, T. C. and Fitzgerald, J. W.: 1985, ‘Incorporation of Sulfate-Sulfur into Organic Matter Extracts of Litter and Soil: Involvement of ATP Sulfurylase,’Soil Biol. Biochem. 17, 779–784.
Tabatabai, M. A. and Bremner, J. M.: 1970, ‘An Alkaline Oxidation Method for Determining Total Sulfur in Soils,’Soil Sci. Soc. Am. Proc. 34, 62–65.
Ueda, A. and Krouse, H. R.: 1986, ‘Direct Conversion of Sulphide and Sulphate Minerals to SO2 for Isotope Analyses,’Geochem. J. 20, 209–212.
Watwood, M. E. and Fitzgerald, J. W.: 1988, ‘Sulfur Transformations in Forest Litter and Soils: Results of Laboratory and Field Incubations,’Soil. Sci. Soc. Am. J. 52, 1478–1483.
Yanagisawa, F. and Sakai, H.: 1983, ‘Precipitation of SO2 for Sulphur Isotope Ratio Measurements by the Thermal Decomposition of BaSO4-V2O5-SiO2 Mixtures,’Anal. Chem. 55, 985–987.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prietzel, J., Mayer, B., Krouse, H.R. et al. Transformation of simulated wet sulfate deposition in forest soils assessed by a core experiment using stable sulfur isotopes. Water Air Soil Pollut 79, 243–260 (1995). https://doi.org/10.1007/BF01100440
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01100440