Abstract
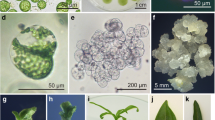
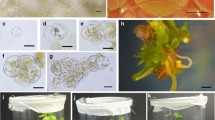
A fast-growing, small, granular, embryogenic callus was selected from primary calli induced from the Japanese wheat cultivar Nakasoushu and the Australian wheat cultivar Bodallin. Regenerable and fine suspension cultures were induced three to six months after liquid culture was initiated and were characterized by dense cytoplasm and active division. These suspension cultures routinely provided high yields of protoplasts with about 90% viability when incubated in a modified KMP (Kao and Michayluk, 1975) medium containing 1 mg l-1 2,4-D (2,4-dichlorophenoxyacetic acid), and 1 mg l-1 zeatin. Nakasoushu and Bodallin protoplasts divided at frequencies of 8.6% and 11.1%, respectively, in agarose-solidified media. When Nakasoushu protoplasts were cultured with effective nurse cells of sorghum and wheat, protoplast division increased to 16.9% and 12.6%, respectively. Plating efficiencies varied from 0.03% to 2.5%. After subculture, protocolonies yielded embryogenic calli and somatic embryos, from which green plants were eventually regenerated. Whole plants obtained from Nakasoushu protoplasts were fertile, demonstrating the first report of Japanese cultivars in wheat protoplast cultures.
Similar content being viewed by others
References
Ahmed KZ & Sagi F (1993) Culture of and fertile plant regeneration from regenerable embryogenic suspension cell-derived protoplasts of wheat (Triticum aestivum L.). Plant Cell Rep. 12: 175–179
Chu CC, Wang CC, Sun CS, Hsu C, Yin KC, Chu CY, & Bi FY (1975) Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen source. Sci. Sin. 18: 659–668
Gamborg OL, Miller RA & Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158
Gu X & Liang GH (1997) Plantlet regeneration from protoplastderived haploid embryogenic calli of wheat. Plant Cell Tissue Organ Cult. 50: 139–145
Hayashi Y & Shimamoto K (1988) Wheat protoplast culture: embryogenic colony formation from protoplasts. Plant Cell Rep. 7: 414–417
He DG, Yang YM & Scott KJ (1992) Plant regeneration from protoplasts derived from suspensions of wheat (Triticum aestivum cv. Hartog). Plant Cell Rep. 11: 16–19
Kao KN & Michayluk MR (1975) Nutritional requirements for growth ofVicia hajastana cells and protoplasts at very low population density in liquid media. Planta. 126: 105–110
Machii H, Mizuno H, Hirabayashi T, Li H & Hagio T (1998) Screening wheat genotypes for high callus induction and regeneration capability from anther and immature embryo cultures. Plant Cell Tiss. Org. Cult. 53:67–74
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Pauk J, Kertesz Z, Jenes B, Purnhauser L, Manninen O, Pulli S, Barabas Z & Dudits D (1994) Fertile wheat (Triticum aestivum L.) Regenerants from Protoplasts of embryogenic suspension culture. Plant Cell Tiss. Org. Cult. 38: 1–10
Shimada T (1991) Cell culture and breeding in wheat (in Japanese). In: Yamada Y and Okada Y (eds.) Plant Biotechnology II(pp. 108–112). Tokyo Kagaku Dojin Press, Tokyo
Shimada T (1992) Practice of tissue and cell cultures in cereal crops (in Japanese). In: Nishi S et al. (eds) In vitro propagation and breeding in cereals, sweet potato and forages (pp. 91–117). Nougyo Tosho Ltd. Press, Tokyo
Takahashi I, Mori S & Yamagata H (1988) Varietal differences of regeneration and protoplast isolation from immature-embryoderived-calluses in common wheat (Triticum aestivum L). Japan J. Breed. 38(2): 190–191
Takamura Y, Otani M, Handa T & Shimada T (1991) Protoplast culture and stable transformation by electroporation in wheat (in Japanese). Japan J. Breed. 41 (Supp. 1): 76–77
Vasil V, Redway F & Vasil IK (1990)Regeneration of plants from embryogenic suspension culture protoplasts of wheat (Triticum aestivum L.). Bio/Technology 8: 429–434
Wang HB, Li XH, Sun YY, Chen J, Zhu Z, Fang R & Wang P (1989) High frequency of callus formation and plant regeneration from protoplasts of wheat (Triticum aestivum L.). Sci. Sin. 8: 828–834
Wang WC & Nguyen NT (1990) A novel approach for efficient plant regeneration from long-term suspension culture of wheat. Plant Cell Rep. 8: 639–642
Wu C & Zapata FJ (1992) Plant regeneration from protoplasts isolated from primary callus of four japonica rice (Oryza sativa L.) varieties. Plant Sci. 86: 83–87
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, H., Machii, H., Hagio, T. et al. Plant regeneration from protoplasts of Triticum aestivum L. cv. Nakasoushu. Plant Cell, Tissue and Organ Culture 58, 119–125 (1999). https://doi.org/10.1023/A:1006359511519
Issue Date:
DOI: https://doi.org/10.1023/A:1006359511519