Abstract
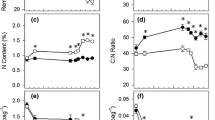
A root decomposition study using the litterbag approach was conducted along a dune and swale chronosequence on the Virginia Coast Reserve-Long Term Ecological Research Site in Virginia, USA to evaluate how environmental and substrate quality factors influence belowground decay and associated nutrient dynamics. Gradients in moisture levels and nitrogen availability associated with the chronosequence provided the experimental framework. Spartina patens roots were buried at all sites as a standard substrate to evaluate environmental influences. Roots native to each site were buried to evaluate community decay dynamics and the influence of litter quality. Spartina decay was reduced in the wet, anoxic soils of swale sites (k = 0.21–0.33 yr-1) relative to decay in dunes soils (k = 0.52–0.72 yr-1). Increasing soil nitrogen availability from younger to older sites had no effect on the rate of Spartina root decay. Native root decay across the Hog Island chronosequence exhibits certain trends expected in response to nitrogen limitation and moisture availability. Increased nitrogen content of root material corresponds to increased soil nitrogen availability. Among dune sites, native root decay increased in concert with increased root nitrogen (6 year k = 0.34 yr-1, 120 year dune: k = 0.97 yr-1). Litter quality, alone, does not explain this trend since Spartina roots decayed more slowly than native dune roots and had a higher initial nitrogen content. Among swales, increased moisture levels and associated soil anoxia inhibited native root decomposition and minimized the effects of litter quality on decay. In general, phosphorus was rapidly lost from decaying roots while nitrogen immobilization was low to nonexistent. The low nitrogen immobilization of decaying roots in a nitrogen limited ecosystem warrants further study and may reveal that belowground decay increases the rate of nutrient cycling relative to decay aboveground.
Similar content being viewed by others
References
Aber J D Melillo J M and McClaugherty C 1990 Predicting longterm patterns of mass, N dynamics and SOM formation from initial fine litter chemistry in temperate forest ecosystems. Can. J. Bot. 68, 2201–2208.
Andren O Lindberg T Bostrum U, et al. 1990 Organic carbon and nitrogen flows. In Ecology of Arable Land. Ecological Studies No. 40. Eds. O Andren, T Lindberg, K Paustian and T Rosswall. pp 85–126. Munksgaard International Publications, Copenhagen.
Barbour MG Burk J H and Pitts WD 1987 Terrestrial Plant Ecology, 2nd ed. Benjamin Cummings, California, USA.
Bargali S S Singh S P and Singh R P 1993 Patterns of weight loss and nutrient release from decomposing litter in an age series of eucalypt plantations. Soil Biol. Biochem. 25, 1731–1738.
Berendse F Berg B and Bosatta E 1987 The effect of lignin and nitrogen on the decomposition of litter in nutrient-poor ecosystems: a theoretical approach. Can. J. Bot. 65, 1116–1120.
Berg B Ekbohm G and McClaugherty C 1984 Lignin and holocellulose relations during long-term decomposition of some forest litters. Long-term decomposition in a Scots pine forest. IV. Can. J. Bot. 62, 2540–2550.
Berg B and McClaugherty C 1989 Nitrogen and phosphorus release from decomposing litter in relation to the disappearance of lignin. Can. J. Bot. 67, 1148–1156.
Blum L K 1993 Spartina alterniflora root dynamics in a Virginia marsh. Mar. Ecol. Prog. Ser. 102, 169–178.
Borie F and Zunino H 1983 Organic matter-phosphorus associations as a sink in P-fixation processes in allophanic soils of Chile. Soil Biol. Biochem 15, 599–603.
Bowman W D Theodose T A Schardt J D and Conant R T 1993 Constraints of nutrient availability on primary production in two alpine tundra communities. Ecology 74, 2085–2097.
Brinson M M Lugo A E and Brown S 1981 Primary productivity, decomposition and consumer activity in freshwater wetlands. Ann. Rev. Ecol. Syst. 12, 123–161.
Camiré C, Côté B and Brulotte S 1991 Decomposition of roots of black alder and hybrid poplar in short-rotation plantings: nitrogen and lignin control. Plant and Soil 138, 123–132.
Chamie J P M and Richardson C J 1978 Decomposition in northern wetlands. In Freshwater Wetlands: Ecological Processes and Management Potential. Eds. R E Good, D F Whigham, and R L Simpson. pp 115–130. Academic Press, New York.
Chapin F S 1980 Themineral nutrition of wild plants. AnnualReview of Ecology and Systematics 11, 233–260.
Conn C E and Day F P 1995 Response of root and cotton strip decay to nitrogen amendment along a barrier island dune chronosequence. Can. J. Bot. 74, 276–284.
Cuevas E and Medina E 1988 Nutrient dynamics within amazonian forests. II. Fine root growth, nutrient availability and leaf litter decomposition. Oecologia 76, 222–235.
Day F P 1995 Environmental influences on belowground decomposition on a coastal barrier island determined by cotton strip assay. Pedobiologia 39, 289–303.
Day Jr F P 1982 Litter decomposition rates in the seasonally flooded Great Dismal Swamp. Ecology 63, 670–678.
Day Jr F P and Megonigal J P 1993 The relationship between variable hydroperiod, production allocation, and belowground organic turnover in forested wetlands. Wetlands 13, 115–121.
Day Jr F P Megonigal J P and Lee L C 1989 Cypress root decomposition in experimental wetland mesocosms.Wetlands 9, 263–282.
Dilustro J J and Day F P 1997 Aboveground biomass and net primary production along aVirginia barrier island dune chronosequence. Am. Midl. Nat. 137, 27–38.
Donnelly P K Entry J A Crawford D L and Cromack Jr K 1990 Cellulose and lignin degradation in forest soils: response to moisture, temperature and acidity. Micro. Ecol. 20, 289–295.
Dueser R D Graham M A Hennessy G J McCaffrey C Niederoda A W Rice T W and Williams B 1976 Ecosystem Description: The Virginia Coast Reserve Study. The Nature Conservancy, Arlington, Virginia, USA.
Ehrenfeld J G 1990 Dynamics and processes of barrier island vegetation. Aquatic Sci. 2, 437–480.
Fahey T J Hughes JW Mou P and Arthur MA 1988 Root decomposition and nutrient flux followlng whole-tree harvest ofNorthern hardwood forest. Forest Sci. 34, 744–768.
Gallardo A and Merino J 1993 Leaf decomposition in two Mediterranean ecosystems of southwest Spain: influences of substrate quality. Ecology 74, 152–161.
Gambrell RP and Patrick Jr W H 1978 Chemical andmicrobiological properties of anaerobic soils and sediments. In Plant Life in Anaerobic Environments. Eds. DD Hook and R M M Crawford. pp 375–423. Ann Arbor, Michigan, USA.
Hackney C T 1987 Factors affecting accumulation or loss of macroorganic matter in salt marsh sediments. Ecology 68, 1109–1113.
Hackney C T and de la Cruz A A 1980 In situ decomposition of roots and rhizomes of two tidal marsh plants. Ecology 61, 226–231.
Harmon E M and Hua C 1991 Coarse woody debris dynamics in two old-growth ecosystems. BioScience 41, 604–610.
Hayden B P Dueser R D Callahan J T and Shugart H H 1991 Longterm research at the Virginia Coast Reserve. BioScience 41, 310–318.
Hendrick R L and Pregitzer K S 1993 The dynamics of fine root length, biomass and nitrogen content in two northern hardwood ecosystems. Can. J. For. Res. 23, 2507–2520.
Hunt HW Ingham E R Coleman D C Elliot E T and Reid C P D 1988 Nitrogen limitation of production and decomposition in prairie, mountain meadow and pine forest. Ecology 69, 1009–1016.
James W P T and Theander O 1981 The Analysis of Dietary Fiber in Food. Marcel Dekker, New York, New York, USA.
Koske R E and Polson W R 1984 Are VA mycorrhizae required for sand dune stabilization? BioScience 34, 420–424.
McClaugherty C A Aber J D and Melillo J M 1982 The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 63, 1481–1490.
McLachlan A and van der Merwe D 1991 Litter decomposition in a coastal dune slack. J. Coastal Res. 7, 107–112.
Meentenmeyer V 1978 Macroclimate and lignin control of litter decomposition rates. Ecology 59, 465–472.
Megonigal J P and Day F P 1988 Organic matter dynamics in four seasonally flooded forest communities of the Dismal Swamp. Am. J. Bot. 75, 1334–1343.
Melillo J M, Aber J D and Muratore J F 1982 Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63, 621–626.
Melillo JM and Aber J D 1984 Nutrient immobilization in decaying Iitter: an example of carbon-nutrient interactions. In Trends in Ecological Research for the 1980s. Eds. J H Cooley and F B Golley. pp 193–215. Plenum Press, New York.
Nadelhoffer KJ Aber JD and Melillo JM 1985 Fine roots, net primary production and soil nitrogen availability: a new hypothesis. Ecology 66, 1377–I390.
Neckles H A and Neill C 1994 Hydrologic control of litter decomposition in seasonally flooded prairie marshes. Hydrobiologia 286, 155–165.
Olff H Huisman J and Van Tooren B F 1993 Species dynamics and nutrient accumulation during early primary succession in coastal sand dunes. J. Ecol. 81, 693–706.
Oosting H J 1945 Tolerance to salt spray of plants of coastal dunes. Ecology 26, 85–89.
Parton W J Schimel D S Cole C V and Ojima D S 1987 Analysis of factors controlling soil organic matter levels in the Great Plains grasslands. Soil Sci. Soc. Am. J. 51, 1173–1179.
Prescott C E Taylor B R Parsons W F J Durall D M and Parkinson D 1993 Nutrient release from decomposing litter in Rocky Mountain coniferous forests: influence of nutrient availability. Can. J. For. Res. 23, 1576–1586.
Rustad L E 1994 Element dynamics along a decay continuum in a red spruce ecosystem in Maine, USA. Ecology 75, 867–879. SAS Institute 1985 SAS Statistics. SAS Institute, Cary, North Carolina, USA.
Schomberg H H Steiner J L and Unger P W 1994 Decomposition and nitrogen dynamics of crop residues: residue quality and water effects. Soil Sci. Soc. Am. J. 58, 372–381.
Seastedt T R 1988 Mass, nitrogen and phosphorus dynamics in foliage and root detritus of tall grass prairie. Ecology 69, 59–65.
Seastedt T R Parton W J and Ojima D S 1992 Mass loss and nitrogen dynamics of decaying litter of grasslands: the apparent low nitrogen immobilization potential of root detritus. Can. J. Bot. 70, 384–391.
Shaver G R and Melillo J M 1984 Nutrient budgets of marsh plants: efficiency concepts and relation to availability. Ecology 65, 1491–1510.
Sokal R R and Rohlf F J 1981 Biometry. W.H. Freeman and Company, New York.
Stump L M and Binkley D 1993 Relationships between litter quality and nitrogen availability in Rocky Mountain forests. Can. J. For. Res. 23, 492–502.
Taylor B R Parkinson D and Parsons W F J 1989 Nitrogen and lignin as predictors of litter decay rates: a microcosm test. Ecology 70, 97–104.
Technicon Industrial Systems 1977 Individual/simultaneous determination of nitrogen and phosphorus in BD acid digests. Iudustrial method No. 329–74w/B, Technicon Industrial Systems, New York.
Tiffney Jr W N and Eveleigh D E 1983 Nitrogen-fixing plants for coastal management. Coastal Zone '83, 102–11.
Tupacz E G and Day F P 1989 Decomposition of roots in a seasonally flooded swamp ecosystem. Aquatic Bot. 37, 199–214.
van der Valk A G 1974 Mineral cycling in coastal foredune plant communities in Cape Hatteras National Seashore. Ecology 55, 1349–1358.
Vitousek P M Gosz J R Grier C C Melillo J M and Reiners W A 1982 Acomparative analysis of potential nitrification and nitrate mobility in forest ecosystems. Ecol. Mon. 52, 155–177.
Vitousek P M and Sanford R L 1986 Nutrient cycling in a moist tropical forest. Ann. Rev. Ecol. Syst. 17, 137–168.
Vitousek P M Turner D R Parton W J and Sanford R L 1994 Litter decomposition on the Mauna Loa environmental matrix, Hawai'i: patterns, mechanisms and models. Ecology 75, 418–429.
Vogt K A Grier C C and Vogt D J 1986 Production, turnover and nutrient dynamics of above-and belowground detritus of world forests. Adv. Ecol. Res. 15, 303–378.
Wieder R K and Lang G E 1982 A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63, 1636–1642. Section editor: R Merckx
Rights and permissions
About this article
Cite this article
Conn, C.E., Day, F.P. Root decomposition across a barrier island chronosequence: Litter quality and environmental controls. Plant and Soil 195, 351–364 (1997). https://doi.org/10.1023/A:1004214216889
Issue Date:
DOI: https://doi.org/10.1023/A:1004214216889