Abstract
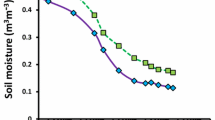
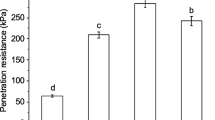
We studied the possibility whether the initiation of secondary roots is regulated by the air-filled porosity in soil, i.e. the availability of oxygen in the soil. Maize plants were grown in long PVC tubes (1 m long and 12 cm diameter) and were unwatered for different numbers of days so that variations of soil water content with depth were achieved on the same date with plants at the same age. The plants were harvested when their root systems were established in the whole soil column and watering had been withheld for 0, 15, 20, 25 days. A decrease of soil water content was significantly correlated with an increase of air-filled porosity in soil. The number of secondary lateral roots from segments of primary adventitious roots increased dramatically when soil water content decreased from field capacity to about 0.05 g water g-1 dried soil. The total dried mass of roots at different soil depths was also positively correlated with soil air-filled porosity. It was observed that the elongation of the initiated secondary roots responded differently to the variations of soil air-filled porosity. The length of secondary roots increased initially when the soil was dried from field capacity to 0.18 g g-1 dried soil (water potential at about−0.2 MPa, air-filled porosity 0.26 cm3 cm-3), but was drastically reduced when the soil was dried further. Obviously elongation of secondary roots was inhibited when soil water potential began to deviate substantially from an optimum value.
The present results suggested that the initiation of secondary roots was greatly promoted by the increase of air-filled soil porosity, i.e. availability of oxygen. This conclusion was further verified in a separate experiment where solution-cultured maize seedlings were subjected to different aeration treatments. An obvious increase in secondary root initiation was found in plants which were aerated with normal air (21% O2) than in plants which were either not aerated or aerated with 5% O2 air. ei]Section editor: B E Clothier
Similar content being viewed by others
References
Armstrong W, Brändle R and Jackson M B 1994 Mechanisms of flood tolerance in plants Acta Bot. Neerl. 43, 307–358.
Bertell G and Eliasson L 1992 Cytokinin effects on root growth and possible interactions with ethylene and indole-3-acetic acid. Physiol. Plant. 84, 255–261.
Bingham I J and Farrar J F 1988 Regulation of respiration in roots of barley. Physiol. Plant. 73, 278–285.
Bingham I J and Stevenson E A 1993 Control of root growth: effects of carbohydrates on the extension, branching and rate of respiration of different fractions of wheat roots. Physiol. Plant. 88, 140–158.
Böttger M 1974 Apical dominance in roots ofPisum sativum L. Planta 121, 253–261.
Charlton W A 1991 Lateral root initiation.In Plant Roots: the Hidden Half. Eds. YWaisel, AEshel and UKafkafi. pp 103–128. Marcel Dekker, New York, USA.
Drew M C, Cobb B G, Johnson J R, Andrews D, Morgan P W, Jordan W and He C J 1994 Metabolic acclimation of root tips to oxygen deficiency. Ann. Bot. 74, 281–286.
Eamus D and Jarvis P G 1989 The direct effects of increase in the global atmospheric CO2 concentration on natural and commercial temperate trees and forests. Adv. Ecol. Res. 19, 1–55.
Friend A L, Coleman M D and Isebrands J G 1994 Carbon allocation to root and shoot systems of woody plants.In Biology of Adventitious Root Formation. Eds. T DDavis and B EHaissig. pp 245–273. Plenum Press, New York, USA.
Geisler G and Krutzfeldt B 1983 Investigations into the effects of “nitrogen” on the marphology, dry matter formation, and nutrient uptake efficiency of the root system of maize, spring barley, and field bean varieties, having regard to temperature conditions. I. Root morphology. J. Agron. Crop Sci. 153, 90–104.
Glinski J and Lipiec J 1990 Soil Physical Conditions and Plant Root. CRC Press, Inc., Boca Raton, FL, USA.
Jordan W R, McCrary M and Miller F R 1979 Compensatory effects of crown root system of sorghum. Agron. J. 71, 803–806.
Klepper B, Belford R K and Rickman R W 1984 Root and shoot development in winter wheat. Agron. J. 76, 117–122.
Macisaac S A and Sawhney V K 1990 Protein changes associated with auxin-induced stimulation and kinetin-induced inhibition of lateral root initiation in lettuce (Lactuca sativa) roots. J. Exp. Bot. 41, 1039–1044.
Macisaac S A, Sawhney V K and Pohorecky Y 1989 Regulation of lateral root formation in lettuce (Lactuca sativa) seedling roots. I. Interacting effects of α-naphthalene acetic acid and kinetin. Physiol. Plant. 77, 287–293.
North G B and Nobel P S 1992 Drought-induced changes in hydraulic conductivity and structure in roots ofFerocactus acanthodes andOpuntia ficus-indica. New Phytol. 120, 9–19.
Ober E S and Sharp R E 1994 Proline accumulation in maize (Zea mays L.) primary roots at low water potentials: I. Requirement for increased levels of abscisic acid. Plant Physiol. 105, 981–987.
Pagès L and Pellerin S 1994 Evaluation of parameters describing the root system architecture of field grown maize plants (Zea mays L.) II. Density, length, and branching of first-order laterals roots. Plant and Soil 164, 169–176.
Peterson R L and Peterson C A 1986 Ontogeny and anatomy of lateral roots.In New Root Formation in Plants and Cuttings. Ed. M BJackson. pp 1–30. Martinus Nijhof Publishers, The Hague, the Netherlands.
Picard D, Jordan M O and Trendel R 1985 Rate of appearance of primary roots of maize. I. Detailed study of one cultivar at one site. Agron. 5, 667–676.
Pilet P-E and Barlow P W 1987 The role of abscisic acid in root growth and gravireaction: A critical review. Plant Growth Regul. 6, 217–265.
Russell R S 1977 Plant Root Systems: Their Function and Interaction with the Soil. McGraw-Hill Book Comp., London, UK.
Saglio P H, Drew M C and Pradet A 1988 Metabolic acclimation to anoxia induced by low (2-kPa partial pressure) oxygen pretreatment (hypoxia) in root tips ofZea mays. Plant Physiol. 86, 61–66.
Seiler G J 1994 Primary and lateral root elongation of sunflower seedlings. Environ. Exp. Bot. 34, 409–418.
Sharp R E, Silk W K and Hsiao T C 1988 Growth of the maize primary root at low water potentials. I. Spatial distribution of expansive growth. Plant Physiol. 87, 50–7.
Tomos D and Pritchard J 1994 Biophysical and biochemical control of cell expansion in roots and leaves. J. Exp. Bot. 45, 1721–1731.
Torrey J G 1986 Endogenous and exogenous influences on the regulation of lateral root formation.In New Root Formation in Plants and Cuttings. Ed. M BJackson. pp 31–66. Martinus Nijhoff Publishers, The Hague, the Netherlands.
Vincent C D and Gregory P J 1989 Effects of temperature on the development and growth of winter wheat roots. I. Controlled glasshouse studies of temperature, nitrogen and irradiance. Plant and Soil 119, 87–97.
Voesenek L A C J and Van derVeen R 1994 The role of phytohormones in plant stress: too much or too little water. Acta Bot. Neerl 43, 91–127.
Wightman F, Schneider E A and Thimann K V 1980 Hormonal factors controlling the initiation and development of lateral roots. II. Effects of exogenous growth factors on lateral root formation in pea roots. Physiol. Plant. 49, 304–314.
Wu Y, Spollen W G, Sharp R E, Hetherington P R and Fry S C 1994 Root growth maintenance at low water potentials: Increased activity of xyloglucan endotransglycosylase and its possible regulation by abscisic acid. Plant Physiol. 106, 607–615.
Yoshida S, Bhattacharjee D P and Cabuslay G S 1982 Relationship between plant type and root growth in rice. Soil Sci. Plant Nutr. 28, 473–482.
Zhang J and Davies W J 1989 Abscisic acid produced in dehydrating roots may enable the plant to measure the water status of the soil. Plant Cell Environ. 12, 73–81.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liang, J., Zhang, J. & Wong, M.H. Effects of air-filled soil porosity and aeration on the initiation and growth of secondary roots of maize (Zea mays). Plant Soil 186, 245–254 (1996). https://doi.org/10.1007/BF02415520
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02415520