Abstract
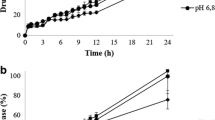
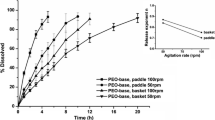
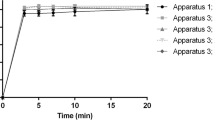
In vitro dissolution profiles of three controlled-release mesalazine formulations were determined at pH 1.0, 6.0 and 7.5. A closed-column type dissolution apparatus was used. A reproducible gradual dissolution profile was seen for Pentasa® at all pH values. Dissolution starts immediately and is complete after 20 h. Dissolution profiles at pH 1 and pH 7.5 are much alike and dissolution is faster than at pH 6. The behaviour of Asacol® at different pH values corresponds with the expectations: no release at pH 6 and pH 1, fast release at pH 7.5. Dissolution starts after 1 h and is complete after 3 h. Mesalazine release from Salofalk® tablets at pH 7.5 and pH 6.0 starts after 2 and 3 h, respectively, and is complete after 5 and 10 h. However, after a long lag-time (10 h) mesalazine is also released from Salofalksu® tablets at pH 1 and dissolution is complete after 23 h.
Similar content being viewed by others
References
Jarnerot G. Newer 5-aminosalicylic acid based drugs in chronic inflammatory bowel disease. Drugs 1989;37:73–86.
Anonymous. United States Pharmacopeia XXI. Rockville: United States Pharmacopeial Convention, 1989.
Langenbucher F, Rettig H. Dissolution rate testing with the column method: methodology and results. Drug Dev Ind Pharm 1977;3:241–63.
FIP Working group V Dissolution Tests. Guidelines for dissolution testing of solid oral products. Pharm Ind 1981;43:334–43.
Langenbucher F. Parametric representation of dissolution curves by the RRSBW distribution. Pharm Ind 1976;38:472–7.
Lee EJD, Bang SB. Simple and sensitive high-performance liquid chromatograph assay for 5-aminosalicylic acid and acetylaminosalicylic acid in serum. J Chromatogr 1987;431:300–4.
Terpstra IJ, Bavelaar JF, Klooster NTM, Groenendaal JW, Hespe W.In vitro dissolution of 5-aminosalicylic acid delivering compounds [Abstract]. 13th International Congress of Gastroenterology. Rome, Sept 1988.
Duchateau A, Philipse R, Van der Hoek E, Conemans J. pH influence onin vitro release of 5-ASA (Mesalazine) [Abstract]. Pharm Weekbl [Sci] 1989;11(Suppl E):E10.
Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988;29:1035–41.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stolk, L.M.L., Rietbroek, R., Wiltink, E.H. et al. Dissolution profiles of mesalazine formulationsin vitro . Pharmaceutisch Weekblad Scientific Edition 12, 200–204 (1990). https://doi.org/10.1007/BF01980047
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01980047