Abstract
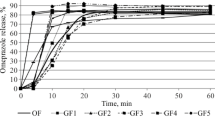
The sustained-release properties and relative bioavailability of Theolin® Retard and Pharphylline® Retard were studied in eight healthy adults after treatment for five days with twice daily 450 mg, respectively 425 mg. During the day-time dosing interval on the fourth and fifth day theophylline plasma concentrations were assayed by HPLC. After intake of Theolin® Retard, minimum theophylline plasma concentrations were significantly higher, fluctuations in theophylline plasma concentrations were significantly smaller andt 75 (the period within a dosing interval during which the plasma concentration exceeds 75% of the maximal concentration) was significantly longer than after Pharphylline® Retard. Maximal concentrations and AUC values were not significantly different. For both products the plasma concentration time-curves on day 5 were significantly lower than on day 4.In vitro dissolution tests confirmed the more sustained release of theophylline from Theolin® Retard. These results indicate an equal extent of absorption from the two products but better sustained-release properties for Theolin® Retard.
Similar content being viewed by others
References
Ellis EF, Koysooko R, Levy G. Pharmacokinetics of theophylline in children with asthma. Pediatrics 1976;58:542–7.
Loughan PM, Sitar DS, Ogilvie RI, Eisen A, Fox Z, Neims AH. Pharmacokinetic analysis of the disposition of intravenous theophylline in young children. J Pediatr 1976;88:874–9.
Katz RM, Rachelefsky GS, Siegel S. The effectiveness of the short- and long-term use of crystallized theophylline in asthmatic children. J Pediatr 1978;92:663–7.
Billing B, Dahlquist R, Garle M, Hörnblad Y, Ripe E. Separate and combined use of terbulatine and theophylline in asthmatics. Effects related to plasma levels. Eur J Respir Dis 1982;63:399–409.
Svedmyr K. Effects of oral theophylline combined with oral and inhaled β2-adrenostimulants in asthmatics. Allergy 1982;37:119–27.
Hendeles L, Weinberger M. Theophylline, a ‘state of the art’ review. Pharmacotherapy 1983;3:2–44.
Kunkel G, Schupp J, Borner K, Lasius D, Meysel U. Untersuchungen zur Theophylline-Wirkung unter besonderer Berücksichtigung der Tagesrhythmik. Allergologie 1983;6:249–55.
Bell T, Bigley J. Sustained-release theophylline therapy for chronic childhood asthma. Pediatrics 1978;62:352–8.
Simons FER, Simons KJ. Pharmacokinetics of theophylline in infancy. J Clin Pharmacol 1978;18:472–6.
Rosen JP, Danish M, Ragni MC, Saccar CL, Yaffe SJ, Lecks HI. Theophylline pharmacokinetics in the young infants. Pediatrics 1979;64:248–51.
Scott PH, Tabachnik E, MacLeod S, Correia J, Newth C, Levison H. Sustained release theophylline for childhood asthma: evidence for circadian variation of theophylline pharmacokinetics. J Pediatr 1981;99:476–9.
Weinberger M, Hendeles L, Wong L. Relationship of formulation and dosing interval to fluctuation of serum theophylline concentration in children with chronic asthma. J Pediatr 1981;99:145–52.
Simons FER, Luciuk GH, Simons KS. Sustained-release theophylline for treatment of asthma in preschool children. Am J Dis Child 1982;136:790–3.
Tabachnik E, Scott P, Correia J, et al. Sustained-release theophylline: a significant advance in the treatment of childhood asthma. J Pediatr 1982;100:489–92.
Jonkman JHG, Schoenmaker R, Greving JE, De Zeeuw RA. Rapid and selective theophylline serum and saliva assay by means of high pressure liquid chromatography. Pharm Weekbl [Sci] 1980;2:49–53.
Denlinger Cl, Stryker KK, Slusher LB, Vesell ES. Studies on theophylline metabolism: autoinduction and inhibition by antipyrine. Clin Pharmacol Ther 1987;41:522–30.
Jonkman JHG, Smolensky MH. Chronopharmacology of theophylline, with special reference to age. In: Reinberg A, Smolensky MH, Lebrecqué G, eds. Biological rhythms and medications. Oxford: Pergamon Press, 1984:73–6. (Annual review of chronopharmacology. Vol. 1).
Jonkman JHG, Van der Boon WJV, Balant LP, Schoenmaker R, Holtkamp A. Chronopharmacokinetics of theophylline after sustained-release and intravenous administration to adults. Eur J Clin Pharmacol 1984;26:215–22.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jonkman, J.H.G., Van Der Boon, W.J.V. & Grasmeijer, G. Comparison of the in vitro dissolution properties and in vivo steady-state pharmacokinetics of two sustained-release theophylline preparations. Pharmaceutisch Weekblad Scientific Edition 10, 17–21 (1988). https://doi.org/10.1007/BF01966430
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01966430