Abstract
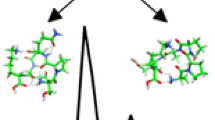
We have synthesized by solution methods and fully characterizedtwo sets of terminally protected peptides based on the tricyclic Cα α-disubstituted glycine Afc. Theconformational preferences of the Afc/Gly peptides were examined by FT-IR absorption and 1H NMR techniques, whilethose of the Afc/TOAC peptides were primarily investigated by using fluorescence spectroscopy. The X-ray diffraction structure of an Afc derivative was also analyzed. The body of solution and crystal-state experimental data conclusively confirms previous findings that the Afc residue may either adopt the fully extended (C5) or a turn conformation.
Similar content being viewed by others
References
Naydenova, E.D., Popova, J.S. and Aleksiev, B.V., In Ramage, R. and Epton, R. (Eds.), Peptides 1996, Mayflower Scientific, Kingswinford, U.K., 1998, pp. 675–676.
Savrda, J., Mazaleyrat, J.-P., Wakselman, M., Formaggio, F., Crisma, M. and Toniolo, C., J. Pept. Sci., 5 (1999) 61.
Toniolo, C., Crisma, M., Formaggio, F., Savrda, J., Mazaleyrat, J.-P. and Wakselman, M., In Bajusz, S. and Hudecz, F. (Eds.), Peptides 1998, Akadémiai Kiadó, Budapest, 1999, pp. 390–391.
DuPriest, M.T., Conrow, R.E. and Kuzmich, D., Tetrahedron Lett., 31 (1990) 2959.
Toniolo, C., CRC Crit. Rev. Biochem., 9 (1980) 1.
Yamada, T., Makihira, K., Suzuki, S., Yoneda, K., Yanagihara, R. and Miyazawa, T., In Shimonishi, Y. (Ed.), Peptide Science: Present and Future, Kluwer, Dordrecht, The Netherlands, 1999, pp. 300–302.
Yamada, T., Makihira, K., Suzuki, S., Yanagihara, R. and Miyazawa, T., In Bajusz, S. and Hudecz, F. (Eds.), Peptides 1998, Akadémiai Kiadó, Budapest, 1999, pp. 404–405.
Marshall, G.R., In Kharasch, N. (Ed.), Intra-Science Chemistry Reports, Gordon and Breach, New York, NY, 1971, pp. 305–316.
Karle, I.L. and Balaram, P., Biochemistry, 29 (1990) 6747.
Toniolo, C. and Benedetti, E., Macromolecules, 24 (1991) 4004.
Toniolo, C., Crisma, M., Fabiano, N., Melchiorri, P., Negri, L., Krause, J.A. and Eggleston, D.S., Int. J. Pept. Protein Res., 44 (1994) 85.
Pavone, V., Lombardi, A., Saviano, M., Nastri, F., Zaccaro, L., Maglio, O., Pedone, C., Omote, Y., Yamanaka, Y. and Yamada, T., J. Pept. Sci., 4 (1998) 21.
Toniolo, C., Crisma, M. and Formaggio, F., Biopolymers (Pept. Sci.), 47 (1998) 153.
Pispisa, B., Palleschi, A., Stella, L., Venanzi, M. and Toniolo, C., J. Phys. Chem., B 102 (1998) 7890.
Sheldrick, G.M., SHELXS 86. Program for the Solution of Crystal Structures, University of Göttingen, Göttingen, Germany, 1986.
Sheldrick, G.M., SHELXL 93. Program for Crystal Structure Refinement, University of Göttingen, Göttingen, Germany, 1993.
Belleau, B. and Malek, G., J. Am. Chem. Soc., 90 (1968) 1651.
Nakaje, C.R., Schreier, S. and Paiva, A.C.M., Biochim. Biophys. Acta, 742 (1983) 63.
Carpino, L.A., J. Am. Chem. Soc., 115 (1993) 4397.
Chakrabarti, P. and Dunitz, J.D., Helv. Chim. Acta, 65 (1982) 1555.
Schweizer, W.B. and Dunitz, J.D., Helv. Chim. Acta, 65 (1982) 1547.
Allen, F.H., Kennard, O., Watson, D.G., Brammer, L., Orpen, A.G. and Taylor, R., J. Chem. Soc., Perkin Trans. 2, (1987) S1.
Valle, G., Crisma, M. and Toniolo, C., Can. J. Chem., 66 (1988) 2575.
IUPAC-IUB Commission on Biochemical Nomenclature, Biochemistry, 9 (1970) 3471.
Bonora, G.M., Mapelli, C., Toniolo, C., Wilkening, R.R. and Stevens, E.S., Int. J. Biol. Macromol., 6 (1984) 179.
Crisma, M., Formaggio, F., Toniolo, C., Yoshikawa, T. and Wakamiya, T., J. Am. Chem. Soc., 121 (1999) 3272.
Crisma, M., Bonora, G.M., Toniolo, C., Benedetti, E., Bavoso, A., Di Blasio, B., Pavone, V. and Pedone, C., Int. J. Biol. Macromol., 10 (1988) 300.
Kopple, K.D. and Ohnishi, M., Biochemistry, 8 (1969) 4087.
Martin, D. and Hauthal, H.G. (Eds.), Dimethyl Sulphoxide, Van Nostrand-Rheinhold, Wokingham, U.K., 1975.
Kennedy, D.F., Crisma, M., Toniolo, C. and Chapman, D., Biochemistry, 30 (1991) 6541.
Venkatachalam, C.M., Biopolymers, 6 (1968) 1425.
Rose, G.D., Gierasch, L.M. and Smith, J.P., Adv. Protein Chem., 37 (1985) 1.
Toniolo, C. and Benedetti, E., Trends Biochem. Sci., 16 (1991) 350.
Mezzato, S., Chemistry Degree Thesis, Department of Organic Chemistry, University of Padova, Italy, 1999.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crisma, M., Formaggio, F., Mezzato, S. et al. Afc can adopt either the fully extended or a turn conformation. Letters in Peptide Science 7, 123–131 (2000). https://doi.org/10.1023/A:1008915732443
Issue Date:
DOI: https://doi.org/10.1023/A:1008915732443