Abstract
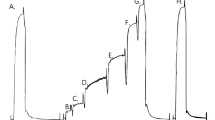
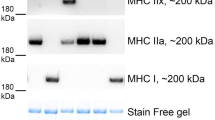
Mechanical properties of myofibrillar bundles from single chemically skinned fibres from the superficial abdominal flexor muscle of the Norway lobster Nephrops norvegicus were measured, and the protein content of these fibres was analysed by SDS-PAGE. Two slow fibre phenotypes (S1, S2) were distinguished on the basis of their myofibrillar protein assemblages. Data from 9 S1 and 8 S2 fibres obtained at similar sarcomere length demonstrate significant differences between the fibre types in maximal tension (N cm-2, S1: 10.5 ± 3.9; S2: 3.1 ± 0.8), in the delay of the peak of stretch activation (ms, S1: 122 ± 18; S2: 412 ± 202), in fibre stiffness (N cm-2 per nm half sarcomere, S1: 0.36 ± 0.19; S2: 0.09 ± 0.03) and in maximal shortening velocity (fibre length s-1, S1: 0.53 ± 0.10; S2: 0.27 ± 0.06). Furthermore, the maximal power output of the type S1 fibres was about five times larger than that of S2 fibres. The power output was maximal at lower loads in S1 fibres (relative load = 0.37 ± 0.04) than in S2 fibres (relative load = 0.44 ± 0.05). This study represents a comprehensive investigation of two slow muscle fibre types which are thought to be specialized for slow movements (S1 fibres) and for the postural control of the abdomen (S2 fibres).
Similar content being viewed by others
References
Altringham JD and Johnston IA (1982) The pCa-tension and force-velocity characteristics of skinned fibres isolated from fish fast and slow muscle fibres. J Physiol 333: 421–449.
Barany M (1967) ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol 50: 197–218.
Bottinelli R, Betto R, Schiaffino S and Reggiani C (1994) Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. J Physiol 478: 341–349.
Bottinelli R, Canepari M, Pellegrino MA and Reggiani C (1996) Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 495: 573–586.
Bremel RD and Weber A (1972) Cooperation within actin filament in vertebrate skeletal muscle. Nature New Biology 238: 97–101.
Brenner B (1988) Effect of Ca2+ on cross-bridge turnover kinetics in single rabbit psoas fibre, implications for the regulation of muscle contraction. Proc Natl Acad Sci USA 85: 3265–3269.
Chase PB and Kushmerick MJ (1988) Effects of pH on contraction of rabbit fast and slow skeletal muscle fibres. Biophys J 53: 935–946.
Eisenberg E, Hill TL and Chen Y (1980) Cross-bridge model of muscle contraction. Biophys J 29: 195–227.
Fowler WS and Neil DM (1992) Histochemical heterogeneity of fibres in the abdominal superficial flexor muscles of the Norway lobster Nephrops norvegicus (L.). J Exp Zool 264: 406–418.
Galler S (1994) Stretch activation of skeletal muscle fibre types. Pflügers Arch 427: 384–386.
Galler S, Schmitt TL and Pette D (1994) Stretch activation, unloaded shortening velocity, and myosin chain isoforms of rat skeletal muscle fibres. J Physiol 478: 513–521.
Galler S, Hilber K and Pette D (1996) Force responses following stepwise length changes of rat skeletal muscle fibre types. J Physiol 493: 219–227.
Galler S and Rathmayer W (1992) Shortening velocity and force/pCa relationship in skinned crab muscle fibres of different types. Pflügers Arch 420: 187–193.
Galler S and Hilber K (1994) Unloaded shortening of skinned mammalian skeletal muscle fibres: effects of the experimental approach and passive force. J Muscle Res Cell Motil 15: 400–412.
Galler S and Neil DM (1994) Calcium activated and stretch induced force responses in two biochemically defined muscle fibre types of the Norway lobster. J Muscle Res Cell Motil 15: 390–399.
Godt RE and Maughan DW (1977) Swelling of skinned muscle fibres of the frog. Biophys J 19: 103–116.
Govind CK, Quigley MM and Mearow KM (1986) The closer muscle in the dimorphic claws of male fiddler crabs. Biol Bull 170: 481–493.
Güth K and Potter JD (1987) Effect of rigor and cycling cross-bridges on the structure of troponin-C and on the calcium affinity of the calcium specific regulatory sites in skinned rabbit psoas fibres. J Biol Chem 262: 13627–13635.
Hilber K and Galler S (1998) Improvement of the measurements on skinned muscle fibres by fixation of the fibre ends with gluteral-dehyde. J Muscle Res Cell Motil (in press).
Huxley HE, Stewart A, Sosa H and Irving T (1994) X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys J 67: 2411–2421.
Kawai M and Brandt PW (1980) Sinusoidal analysis: high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil 1: 1279–1303.
Kawai M and Schachat H (1984) Differences in the transient response of fast and slow skeletal muscle fibres. Correlations between complex modules and myosin light chains. Biophys J 45: 1145–1151.
Kawai M and Zhao Y (1993) Cross-bridge scheme and force per cross-bridge state in skinned rabbit psoas muscle fibers. Biophy J 65: 638–651.
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227: 680–685.
Lännergren J (1978) The force-velocity relation of isolated twitch and slow muscle fibres of Xenopus leavis. J Physiol 283: 501–521.
Leavis PC and Gergely J (1984) Thin filament proteins and thin filament-linked regulation of vertebrate muscle contraction. Crit-ical Rev Biochem 16: 235–305.
Lehman W and Szent-Györgyi AG (1975) Regulation of muscular contraction. Distribution of actin control and myosin control in the animal kingdom. J Gen Physiol 66: 1–30.
Lehman W, Craig R, Vibert P (1994) Ca2+-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature 386: 65–67.
Li Y and Mykles DL (1990) Analysis of myosins from lobster muscles: fast and slow isozymes differ in heavy-chain composition. J Exp Zool 255: 163–170.
Miegel A, Kobayashi I and Maeda Y (1992) Isolation, purification and partial characterisation of tropomyosin and troponin subunits from the lobster tail muscle. J Muscle Res Cell Motil 13: 608–618.
Müller A, Wolf H, Galler S and Rathmayer W (1992) Correlation of electrophysiological, histochemical and contractional properties in fibres of the coxal rotator of the locust, Locusta migratoria. J Comp Physiol B 162: 5–15.
Mykles DL (1985a) Heterogeneity of myofibrillar proteins in lobster fast and slow muscles: Variants of troponin, paramyosin, and myosin light chains comprise four distinct protein assemblages. J Exp Zool 234: 23–32.
Mykles DL (1985b) Multiple variants of myofibrillar proteins in single fibres of lobster claw muscles, Evidence for two types of fibres in the cutter claw. Biol Bull 169: 476–483.
Mykles DL (1988) Histochemical and biochemical characterisation of two slow fibre subtypes in decapod crustacean muscles. J Exp Zool 245: 232–243.
Neil DM, Fowler WS and Tobasnick G (1993) Myofibrillar protein composition correlates with histochemistry in fibres of the abdo-minal flexor muscles of the Norway lobster Nephrops norvegicus. J Exp Biol 183: 185–201.
Ohtsuki I, Maeuyama K and Ebashi S (1986) Regulatory and cytoskeletal proteins of vertebrate skeletal muscle. Adv Protein Chem 38: 1–67.
Parnas I and Atwood HL (1966) Phasic and tonic neuromusclar systems in the abdominal extensor muscles of the crayfish and rock lobster. Comp Biochem Physiol 18: 701–723.
Quigley MM and Mellon DeF (1984) Changes in myofibrillar gene expression during fibre-type transformation in claw closer muscles of the snapping shrimp, Alpheus heterochelis. Dev Biol 106: 262–265.
Reiser PJ, Greaser ML and Moss RL (1988) Myosin heavy chain composition of single cells from avian slow skeletal muscle is strongly correlated with velocity of shortening during develop-ment. Dev Biol 129: 400–407.
Silverman H, Costello WJ and Mykles DJ (1987) Morphological fibre type correlates of physiological and biochemical properties in crustacean muscle. Am Zool 27: 1011–1019.
Wakabayashi K, Sugimoto Y, Tanaka H, Ueno Y, Takezawa Y and Amemiya Y (1994) X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J 67: 2422–2435.
Wang G and Kawai M (1996) Effects of MgATP and MgADP on the cross-bridge kinetics of rabbit soleus slow-twitch muscle fibres. Biophys J 71: 1450–1462.
West JM, Humphris DC and Stephenson DG (1992) Differences in maximal activation properties of skinned short-and long-sarcomere muscle fibres from the claw of the freshwater crustacean Cherax destructor. J Muscle Res Cell Motil 13: 668–684.
Woledge RC, Curtin NA and Homsher E (1985) Energetic aspects of muscle contraction. Academic Press, London.
Zot HG and Potter JD (1987) Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. A Rev Biophys Biophys Chem 16: 535–559.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holmes, J.M., Hilber, K., Galler, S. et al. Shortening properties of two biochemically defined muscle fibre types of the Norway lobster Nephrops norvegicus L.. J Muscle Res Cell Motil 20, 265–278 (1999). https://doi.org/10.1023/A:1005481725344
Issue Date:
DOI: https://doi.org/10.1023/A:1005481725344