Abstract
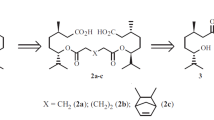
A simple approach is presented whereby omega halogenated fatty acids can be obtained from macrocyclic musk lactones which are industrially available. While providing a secure source of 16-iodo-hexadecanoic acid and 17-iodo-heptadecanoic acid, the scheme allows ready access to a large number of untried fatty acid analogs. Examples presented are 16-iodo-hexadecanoic acid. 16-iodo-7-hexadecenoic acid. 16-iodo-12-oxa-hexadecanoic acid, 15-iodo-pentadecanoic acid, and 15-iodo-12-keto-pentadecanoic acid.
Similar content being viewed by others
References
N. D. POE, G. D. ROBINSON, N. S. MacDONALD, Proc. Soc. Exp. Med., 148 (1975) 215.
G. D. ROBINSON, Intern. J. Appl. Radiation Isotopes, 28 (1977) 149.
H. J. MACHULLA, G. STOCKLIN, C. KUPFERNAGEL et al., J. Nucl. Med., 19 (1978) 298.
H. R. SCHON, H. R. SCHELBERT, G. D. ROBINSON et al., Am. Heart J., 103 (1982) 532.
H. R. SCHON, H. R. SCHELBERT, A. NAJAFI, et al., Am. Heart J., 103 (1982) 548.
H. R. SCHELBERT, E. HENZE, M. E. PHELPS et al., Am. Heart J., 103 (1982) 588.
E. E. VAN DER WALL, Dissertation, Vrije Universiteit Te Amsterdam, 1981.
H. J. MACHULLA, M. MARSMANN, K. DUTSCHKA, Eur. J. Nucl. Med., 5 (1980) 171.
G. W. KABALKA, E. E. GOOCH, C. OTTO, J. Radioanal. Chem., 65 (1981) 115.
G. STOCKLIN, G. KLOSTER, Metabolic Analogue Tracers, in Computed Emission Tomography, P. J. ELL, B. L. HOLMAN (Eds), Oxford U. Press, 1982, p. 299.
H. J. MACHULLA, Radioactive Labelling of Fatty Acids for Metabolic Studies, in Applications of Nuclear and Radiochemistry, R. M. LABRECHT, N. MORCOS (Eds), Pergamon Press, 1982, p. 325.
F. F. KNAPP, M. M. GOODMAN, A. P. CALLAHAN et al., J. Med. Chem., 26 (1983) 1293.
M. ARGENTINI, M. ZAHNER, P. A. SCHUBIGER, J. Radioanal. Chem., 65 (1981) 131.
P. R. STORY, P. BUSCH, Adv. Org. Chem., 8 (1972) 67.
T. G. BACK, Tetrahedron 33 (1977) 3041.
K. C. NICOLAOU, Tetrahedron, 33 (1977) 683.
G. OHLOFF, Fortschr. Chem. Forsch., 12 (1969) 185.
S. ABE, T. ETO, Y. TSUJITO, Koryo, 96 (1970) 19.
S. ABE, T. ETO, Y. TSUJITO, Cosmetics and Perfumery, 88 (1973)67.
S. ABE, Koryo, 135 (1982) 37.
P. Z. BEDOUKAIN, Perfumery and Flavor Synthetics, Second ed., Elsevier, New York, 1967, p. 248.
L. RŮZIĆKA, Helv. Chim. Acta, 9 (1926) 230.
L. RŮZIĆKA, Helv. Chim. Acta, 9 (1926) 715.
M. KERSCHBAUM, Ber., 60B (1927) 902.
E. W. SANAGEL, W. H. CAROTHERS, J. Am. Chem. Soc., 57 (1935) 929.
E. W. SANAGEL, W. H. CAROTHERS, J. Am. Chem. Soc., 58 (1936) 654.
G. OHLOFF, J. BECKER, K. H. SCHULTE-ELTE, Helv. Chim. Acta, 50 (1967) 705.
A. ESCHENMOSER, D. FELIX, G. OHLOFF, Helv. Chim. Acta, 50 (1967) 708.
H. NOZAKI, T. MORE, R. NOYORI, Tetrahedron Letters, 9 (1967) 779.
K. A. BAUER, A. K. KÖRBER, Old and New Macrocyclic Musks: A General Review from the Industrial Standpoint, in Fragrance and Flavor Substances, R. CROTEAU (Ed.) D. and PS Verlag, 3017 Pattensen 1, West Germany, 1980, p. 129.
J. J. BECKER, United States Patent 3,890,353 Issued 17 June 1975.
R. HOPP, K. BAUER, West Germany Patent 24 10 859 Issued 18 September 1975.
F. IMAIZUMI, K. GOTO, K. SEKINE, Japanese Patent 78 90,284. Issued August 8, 1978.
F. IMAIZUMI, K. GOTO, K. SEKINE, Japanese Patent 78 90,283. Issued August 8, 1978.
K. BAUER, A. KÖRBER, K. H. BORK, West Germany Patent 29 29 864, Issued 19 February 1981.
H. HUNSDIECKER, Ber., 76B (1943) 142.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dougan, H., Lyster, D.M. & Vincent, J.S. Macrocyclic lactones as a source for radiohalogenated fatty acid analogs and their precursors. Journal of Radioanalytical and Nuclear Chemistry, Articles 89, 71–78 (1985). https://doi.org/10.1007/BF02070205
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02070205