Abstract
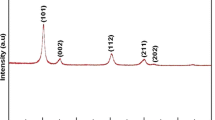
Uniform particles of controlled morphology of CeOSO4·H2O may be prepared by forced hydrolysis, at 90 °C, of solutions of cerium (IV) sulphate. A structural description is given here of the different steps of the forced hydrolysis before precipitation takes place. It uses essentially the complementary techniques of extended X-ray absorption fine structure spectroscopy and small-angle X-ray scattering to characterize, at different length scales, the structural evolution of the solution. The first step, which occurs as temperature is raised above 60 °C, is an inorganic polymerization that transforms molecular dimeric precursors Ce2(OH)2O12 into colloidal particles. In the second step, at the ageing temperature of 90 °C, no chemical and structural changes are revealed; the solution has reached an equilibrium state characterized by the presence of 3 nm large monodisperse colloids which use 85% of the initial cerium ions and smaller particles (15%). The detailed local structure around the cerium atoms in the colloids is compatible with the formation of a chain-like structure of Ce(IV) ions via hydroxo bridges (Ce(OH)2) 2 n+ n . A mechanism to explain the transformation of precursors into colloids is proposed.
Similar content being viewed by others
References
L. L. Hench and D. R. Ulrich (Eds) “Ultrastructure Processing of Ceramics, Glasses and Composites” (Wiley-Interscience, New-York, 1984).
E. Matijevic, Pure Appl. Chem. 60 (1988) 1479.
Ph. Tailhades, P. Mollard, A. Rousset and M. Gougeon, IEEE Trans. Magnetics 26 (1990) 1822.
E. Matijevic, Progr. Colloid. Polym. Sci. 61 (1976) 24.
Idem, Pure Appl. Chem. 52 (1980) 1193.
Idem, Acc. Chem. Res. 14 (1981) 22.
Idem, Ann. Rev. Mater. Sci. 15 (1985) 483.
Idem, Langmuir 2 (1986) 12.
T. Sugimoto, Adv. Colloid Interface Sci. 28 (1987) 65.
Idem, Mater. Res. Soc. Bull. 14 (1989) 23.
M. Haruta and B. Delmon, J. Chim. Phys. 83 (1986) 859.
J. Livage, M. Henry and C. Sanchez, Pure Solid State Chem. 18 (1988) 259.
J. Livage, M. Henry, J. P. Jolivet and C. Sanchez, Mater. Res. Soc. Bull. 14 (1989) 18.
R. Demchak and E. Matijevic, J. Colloid. Interface. Sci. 31 (1969) 257.
E. Matijevic and R. S. Sapieszko, ibid. 50 (1975) 567.
R. S. Sapieszko, R. C. Patel and E. Matijevic, J. Phys. Chem. 81 (1977) 1061.
E. Matijevic and P. Scheiner, J. Colloid Interface. Sci. 63 (1978) 509.
E. Matijevic, M. Budnik and L. Meites, ibid. 61 (1977) 302.
W. B. Scott and E. Matijevic, ibid. 66 (1978) 447.
H. Sasaki, E. Matijevic and E. Barouch, ibid. 76 (1980) 319.
N. B. Milic and E. Matijevic, ibid. 85 (1982) 306.
S. Hamada, K. Bando and Y. Kudo, J. Chem. Soc. Jpn 6 (1984) 1068.
D. H. Buss, G. Schaumberg and O. Glemser, Angew. Chem. 97 (1985) 1050.
M. A. Blesa, A. J. G. Maroto, S. I. Passaggio, N. E. Figliolia and G. Rigotti, J. Mater. Sci. 20 (1985) 4601.
W. P. Hsu, L. Ronnquist and E. Matijevic, Langmuir 4 (1988) 31.
M. Castellano and E. Matijevic, Chem. Mater. 1 (1989) 78.
K. Yura, K. C. Fredrikson and E. Matijevic, Colloid Surf. 50 (1990) 281.
V. Briois, C. E. Williams, H. Dexpert, M. Henry, J. P. Jolivet, F. Deneuve and C. Magnier, in preparation.
V. Briois, C. E. Williams, H. Dexpert, F. Villain, M. Verdaguer, A. Pourpoint, F. Deneuve and C. Magnier, in preparation.
A. H. Kunz, Anal. Chem. 53 (1931) 98.
E. Wadsworth, F. R. Duke and C. A. Goetz, Anal. Chem. 12 (1957) 1824.
J. M. Dubuisson, J. M. Dauvergne, C. Depautex, P. Vachette and C. E. Williams, Nucl. Instrum. Meth. Phys. Res. A246 (1986) 636.
A. Guinier and G. Fournet, “Small Angle Scattering of X-rays” (Wiley, New York, 1955).
O. Glatter, Acta Phys. Aust. 36 (1972) 307.
O. Glatter and O. Kratky (Eds) “Small Angle X-Ray Scattering” (Academic Press, London, 1982).
Ph. Sainctavit, J. Petiau, A. Manceau, R. Rivallant, M. Belakhovsky and G. Renaud, Nucl. Instrum. Meth. Phys. Res. A273 (1988) 423.
P. Lagarde, M. Lemonnier and H. Dexpert, Physica B 158 (1989) 337.
C. Prieto, P. Lagarde, H. Dexpert, V. Briois, F. Villain and M. Verdaguer, Meas. Sci. Technol. 3 (1992) 325.
D. E. Sayers, F. W. Lytle and E. A. Stern, Adv. X-Ray Anal. 13 (1970) 248.
D. E. Sayers, E. A. Stern and F. W. Lytle, Phys. Rev. Lett. 27 (1971) 1204.
F. W. Lytle, D. E. Sayers and E. A. Stern, Phys. Rev. B 11 (1975) 4825.
E. A. Stern, D. E. Sayers and F. W. Lytle, ibid. 11 (1975) 4836.
B. K. Teo, “Inorganic Chemistry Concepts” Vol. 9, “EXAFS: Basic Principles and Data Analysis” (Springer, Berlin, 1986).
A. G. McKale, B. W. Veal, A. P. Paulikas, S. K. Chan and G. S. Knapp, J. Am. Chem. Soc. 110 (1988) 3763.
F. Taulelle, private communication (1991).
V. Briois, J. Lemerle and J. Eberle, in preparation.
T. Svedberg and K. O. Pedersen, “The Ultracentrifuge” (Clarendon Press, Oxford, London, 1940).
G. Champetier, in “Chimie Macromoléculaire”, Vol II (Hermann, Paris, 1972) Ch. V.
L. T. Bugaenko and H. Kuan-Lin, Russ. J. Inorg. Chem. 8 (1963) 1299.
T. N. Bondareva, V. F. Barkovskii and T. V. Velikanova, ibid. 10 (1965) 67.
L. V. Trubacheva and N. I. Pechurova, ibid. 26 (1981) 1745.
O. Lindgren, Acta Chem. Scand. A31 (1977) 163.
G. Lundgren, Arkiv Kemi 10 (1956) 183.
O. Lindgren, Acta Chem. Scand. A31 (1977) 453.
G. V. Trofimov and V. I. Belokoskov, Russ. J. Inorg. Chem. 13 (1968) 135.
W. J. Evans, T. J. Deming, J. M. Olofson and J. W. Ziller, Inorg. Chem. 28 (1989) 4027.
P. S. Gradeff, K. Yunlu, A. Gleizes and J. Galy, Polyhedron 8 (1989) 1001.
D. L. Rogachev, M. A. Porai-Koshits, V. Ya. Kuznetsov and L. M. Dikareva, J. Struct. Chem. 15 (1974) 397.
K. S. Pitzer, R. N. Roy and L. F. Silvester, J. Am. Chem. Soc. 99 (1977) 4930.
M. Hansson, Acta Chem. Scand. 27 (1973) 2455.
M. El Brahimi, J. Durand and L. Cot, Eur. J. Solid State Inorg. Chem. 25 (1988) 185.
G. Lundgren, Arkiv Kemi 2 (1950) 535.
Idem, ibid. 4 (1952) 421.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Briois, V., Williams, C.E., Dexpert, H. et al. Formation of solid particles by hydrolysis of cerium (IV) sulphate. JOURNAL OF MATERIALS SCIENCE 28, 5019–5031 (1993). https://doi.org/10.1007/BF00361172
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00361172