Abstract
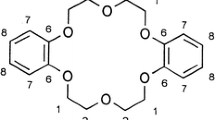
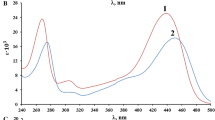
The complex formation reaction between iodine and 1,7-diaza-15-crown-5 (DA15C5) has been studied spectrophotometrically in chloroform at 25°C. The resulting 1:2 (DA15C5:I2) molecular complex was formulated as (DA15C5...;I+)I −3 . The spectrophotometric results, as well as the conductivity measurements, revealed that the gradual release of triiodide ion from its contact ion paired form in the molecular complex into the solution is the rate determining step of the reaction. The rate constant was calculated ask=(8.8±0.2)×10−3 min−1. The formation constant of the molecular complex was evaluated from the computer fitting of the absorbance-mole ratio data as logK f=6.89±0.09.
Similar content being viewed by others
References
M. Brandon, M. Tamres, and S. Searles:J. Am. Chem. Soc. 82, 2129 (1960).
E. M. Arnett and C. Y. Wu:J. Am. Chem. Soc. 84, 1684 (1962).
L. J. Andrews and R. M. Keefer:Molecular Complexes in Organic Chemistry, Holden-Day (1964).
M. Tamres and J. Yarwood:Spectroscopy and Structure of Molecular Complexes, Ch. 3., Plenum Press (1974).
P. J. Trotter and P. A. White:Appl Spectrosc. 32, 232 (1978).
I. Ikemoto, M. Sakairi, T. Tsutsumi, H. Kuroda, I. Tasumi, and H. Shirakawa:Chem. Lett. 1189 (1979).
N. Kulevsky and K. N. Butamina:Spectrochim. Acta 46A, 79 (1991).
R. M. Izatt, J. S. Bradshaw, K. Pawlak, R. L. Bruening, and B. J. Tarbet:Chem. Rev. 92, 1261 (1992).
H. P. Hopkins, D. V. Jahagirdar, and F. J. Windler:J. Phys. Chem. 82 1254 (1978).
L. J. Andrews and R. M. Keefer:J. Org. Chem. 52, 2690 (1987).
E. M. Nour and L. A. Shahada:Spectrochim. Acta 44A, 1277 (1988).
E. M. Nour:Spectrochim. Acta 47A, 743 (1991).
W. Hirsch, J. Greenman, and R. Pizer:Can. J. Chem. 71, 2171 (1993).
A. Semnani and M. Shamsipur:Spectrochim. Acta 49A, 411 (1993).
R. P. Lang:J. Phys. Chem. 78, 1657 (1974).
L. Andrews, E. S. Prochaska and A. LoewenschussInorg. Chem. 19, 463 (1980).
M. Mizuno, J. Tanaka, and I. Harada:J. Phys. Chem. 85, 1789 (1981).
Y. A. Serguchev and T. I. Petrenko:Teor. Eksp. Khim. 13, 705 (1977).
V. A. Nicely and J. L. Dye:J. Chem. Educ. 48, 443 (1971).
W. E. Wentworth:J. Chem. Educ. 42, 96, 162 (1962).
M. J. D. Powell:Comput. J. 7, 155 (1964).
P. Labbe, R. Le Goaller, H. Handel, G. Pierre, and J. L. Pierre:Electrochim. Acta 27, 257 (1982).
R. G. Pearson:Struct. Bonding 80, 1 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Semnani, A., Shamsipur, M. Spectrophotometric study of the complexation of iodine with 1,7-diaza-15-crown-5 in chloroform solution. J Incl Phenom Macrocycl Chem 22, 99–105 (1995). https://doi.org/10.1007/BF00707685
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00707685