Abstract
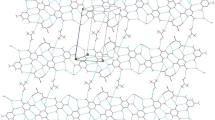
New inclusion complexes R4N+HCO −3 ·x(NH2)2CS·yH2O (1, R=C2H5,x=1,y=1;2, R=n−C3H7,x=2,y=0;3, R=n−C4H9,x=3,y=0) have been prepared and characterized by X-ray crystallography. Crystal data, MoK α radiation:1, space groupPbca,Z=8,a=8.839(2),b=14.930(3),c=24.852(5) Å, andR F=0.063 for 1419 observed data;2, space groupC2221,Z=8,a=8.521(3),b=16.941(4),c=32.022(7) Å,R F=0.054 for 1689 observed data;3, space group\(P\bar 1\),Z=2,a=9.553(2),b=12.313(3),c=14.228(4) Å, α=90.44(2),β=103.11(2), γ=110.12(2)°,R F=0.044 for 3925 observed data. In the crystal structure of1, the thiourea molecules form hydrogen-bonded zigzag ribbons running parallel to thea axis, and the cyclic dimeric bicarbonate moieties (HCO −3 )2 together with water molecules behave likewise. A puckered layer is formed by further lateral hydrogen bonding between these two types of ribbons, and the (C2H5)4N+ cations occupy the space between adjacent layers. In the crystal structure of2, the thiourea ribbons are cross-linked orthogonally by (HCO −3 )2 unitsvia N−H...O hydrogen bonds to form a composite double layer. Half of the cations are enclosed within and the other half sandwiched between these double layers. In the crystal structure of3, the thiourea molecules form puckered double ribbons running in the [110] direction. The host framework is constructed by cross-linking the double ribbons with bridging bicarbonate dimers, yielding two channel systems aligned parallel to [100] and [111] that accommodate the cationic guests. The structural relationship between the present complexes and the classical thiourea channel adducts is discussed.
Similar content being viewed by others
References
K. Takemota and N. Sonoda: in J. L. Atwood, J. E. D. Davies and D. D. MacNicol (Eds.),Inclusion Compounds, Vol. 2, pp. 47–67. Academic Press, London (1984).
L.C. Fetterly: in L. Mandelcorn (Ed.),Non-stoichiometric Compounds, pp. 491–567, Academic Press, New York (1964).
K.D.M. Harris and J.M. Thomas:J. Chem. Soc., Faraday Trans. 86, 2985 (1990); K.D.M. Harris, S.P. Smart, and M.D. Hollingsworth:ibid. 87, 3423 (1991).
K.D.M. Harris:Chem. Brit. 29, 132 (1993).
H.-U. Lenne:Acta Crystallogr. 7, 1 (1954).
E. Hough and D.G. Nicholson:J. Chem. Soc., Dalton Trans. 15 (1978).
K.D.M. Harris and J.M. Thomas:J. Chem. Soc., Faraday Trans. 86, 1095 (1990).
T.C.W. Mak and G.D. Zhou:Crystallography in Modern Chemistry, p. 180, Wiley, New York (1992).
P.A. Schofield, K.D.M. Harris, I.J. Shannon, and A.J.O. Rennie:J. Chem. Soc., Chem. Commun. 1293 (1993).
A.G. Anderson, J.C. Calabrese, W. Tam, and I.D. Williams:Chem. Phys. Lett. 134, 392 (1987).
A.E. Tonelli:Comput. Polym. Sci. 2(2–3), 80 (1992).
R.K. McMullan, T.C.W. Mak, and G.A. Jeffrey:J. Chem. Phys. 44, 2338 (1966).
W.J. McLean and G.A. Jeffrey:J. Chem. Phys. 47, 414 (1967).
W.J. McLean and G.A. Jeffrey:J. Chem. Phys. 49, 4556 (1968).
T.C.W. Mak:J. Incl. Phenom. 4, 273 (1986).
W. Michael, F. Clemens C., F. Juergen, and E. Guenter:Z. Naturforsch., B:Chem. 48, 978 (1993).
T.C.W. Mak:J. Incl. Phenom. 8, 199 (1990). Part I of this series.
R.T. Morrison and R.N. Boyd:Organic Chemistry, 6th edit., p. 854, Prentice-Hall, London (1992).
R.A. Sparks: in F.R. Ahmed (Ed.),Crystallographic Computing Techniques, p. 452. Munksgaard, Copenhagen (1976).
G. Kopfmann and R. Huber:Acta Crystallogr., Sect. A 24, 348 (1968).
G.M. Sheldrick: in D. Sayre (Ed.),Computational Crystallography, Oxford University Press, New York, pp. 506–514 (1982).
International Tables for X-ray Crystallography, Vol. IV, Kynoch Press, Birmingham (1974) (Distrib.: Kluwer Academic Publishers, Dordrecht), pp. 55, 99, 149.
T.C.W. Mak:J. Incl. Phenom. 6, 473 (1988).
S. Swaminathan, B.M. Craven, and R.K. McMullan:Acta Crystallogr., Sect. B 40, 300 (1984).
R. Bishop and I.G. Dance:Top. Curr. Chem. 149, 137 (1988).
V. Philip and D. Jerry:Acta Crystallogr. 5, 530 (1952).
R.L. Sass and R.F. Scheuerman:Acta Crystallogr. 15, 77 (1962).
I. Nitta, Y. Tomiie, and C.H. Koo:Acta Crystallogr. 5, 292 (1952).
Author information
Authors and Affiliations
Additional information
Supplementary Data relating to this article have been deposited with the British Library as Supplementary Publication No. SUP 82178 (44 pages).
Rights and permissions
About this article
Cite this article
Li, Q., Mak, T.C.W. Inclusion compounds of thiourea and peralkylated ammonium salts. Part II. Hydrogen-bonded host lattices built of thiourea and cyclic dimeric bicarbonate moieties. J Incl Phenom Macrocycl Chem 20, 73–88 (1994). https://doi.org/10.1007/BF00707613
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00707613