Abstract
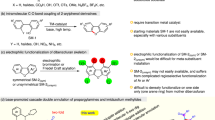
A general study on the synthesis of partly bridged octols3a-d and4c-d is described. Tri-bridged diol3c can be prepared in 54% yield in DMSO at 70°C with excess CH2BrCl or in 52% yield in DMF at 70°C with only 4 equiv. of CH2BrCl. 1,3-Di-bridged tetrol4a, one of the two possible di-bridged isomers formed in preference to the other, was obtained in 30% yield. Tri-bridged diols3c andd can be selectively debrominated in one step by treatment with 5 equiv. ofn-BuLi in THF to afford the corresponding dibromo derivatives8a andb in 77% and 76% yields, respectively. After incorporation of the fourth bridge, the remaining two bromines can be replaced by C(O)OMe to give9c (60%), by OH to give9d (62%) or by CN to give9f (>95%). When the lithiated derivatives of3c andd are quenched with electrophiles other thanH +, a selectively functionalized tri-bridged diol with hydroxyl (8c, 47%) and selectively functionalized cavitands with thiomethyl (9g, 25%) or iodo (9h, 20%) groups can be synthesized. Two molecules of9d were coupled with CH2BrCl in DMSO/THF under high dilution conditions to give the flexible hemicarcerand10 in 71% yield.
Similar content being viewed by others
References
C. D. Gutsche:Calixarenes. Monographs in Supramolecular Chemistry, Vol. 1, J. F. Stoddart (Ed.), The Royal Society of Chemistry, Cambridge (1989).
J Vicens and V. Böhmer (Eds.):Calixarenes: A Versatile Class of Macrocyclic Compounds, Kluwer Academic Publishers, Dordrecht (1991).
C. D. Gutsche and P. F. Pagoria:J. Org. Chem. 50, 5795 (1985). C. D. Gutsche, J. A. Levine, and P. K. Sujeeth:J. Org. Chem. 50, 5802 (1985).
K. No and Y. Noh:Bull. Korean Chem. Soc. 7, 314 (1986).
K. No, Y. Noh, and Y. Kim:Bull. Korean Chem. Soc. 7, 442 (1986).
S. Shinkai, K. Araki, T. Tsubaki, T. Arimura, and O. Manabe:J. Chem. Soc., Perkin Trans. 1 2297 (1987).
M. Almi, A. Arduini, A. Casnati, A. Pochini, and R. Ungaro:Tetrahedron 45, 2177 (1989).
J.-D. van Loon, W. Verboom, and D. N. Reinhoudt:Org. Prep. Proc. Int. 24, 437 (1992).
L. C. Groenen, B. H. M. Rüel, A. Casnati, W. Verboom, A. Pochini, R. Ungaro, and D. N. Reinhoudt,Tetrahedron 39, 8379 (1991).
E. Kelderman, L. Derhaeg, G. J. T. Heesink, W. Verboom, J. F. J. Engbersen, N. F. van Hulst, A. Persoons, and D. N. Reinhoudt:Angew. Chem. Int. Ed. Engl. 31, 1075 (1992).
L. C. Groenen, B. H. M. Rüel, A. Casnati, P. Timmerman, W. Verboom, S. Harkema, A. Pochini, R. Ungaro, and D. N. Reinhoudt:Tetrahedron Lett. 32, 2675 (1991).
S. Pappalardo, L. Giunta, M. Foti, G. Ferguson, J. F. Gallagher, and B. Kaitner:J. Org. Chem. 57, 2611 (1992).
J.-D. van Loon, A. Arduini, L. Coppi, W. Verboom, A. Pochini, R. Ungaro, S. Harkema, and D. N. Reinhoudt:J. Org. Chem. 55, 5639 (1990).
Y. Ting, W. Verboom, L. C. Groenen, J.-D. van Loon, and D. N. Reinhoudt:J. Chem. Soc., Chem. Commun. 1432 (1990).
Z. Goren and S. Biali:J. Chem. Soc., Perkin Trans. 1 1484 (1990).
C. D. Gutsche, B. Dhawan, J. A. Levine, K. H. No, and L. J. Bauer:Tetrahedron 39, 409 (1983).
K. Iwamoto, K. Araki, and S. Shinkai:Tetrahedron 47, 4325 (1991).
H. Erdtman, S. Högberg, S. Abrahamsson, and B. Nilsson:Tetrahedron Lett. 1679 (1968).
D. J. Cram, S. Karbach, H.-S. Kim, C. B. Knobler, E. F. Maverick, J. L. Ericson, and R. C. Helgeson:J. Am. Chem. Soc. 110, 2229 (1988).
D. J. Cram, M. E. Tanner, and C. B. Knobler:J. Am. Chem. Soc. 113, 7717 (1991).
P. Soncini, S. Bonsignore, E. Dalcanale, and F. Ugozzoli:J. Org. Chem. 57, 4608 (1992).
D. J. Cram, M. E. Tanner, and R. Thomas:Angew. Chem. Int. Ed. Engl. 30, 1024 (1991).
Part of this work was published as a preliminary communication: P. Timmerman, M. G. A. van Mook, Mook, W. Verboom, G. J. van Hummel, S. Harkema, and D. N. Reinhoudt:Tetrahedron Lett. 33, 3377 (1992).
T. N. Sorrell and J. L. Richards:Synlett 155 (1992).
Throughout this paper we use the nomenclature proposed by Cram [35]:starting from a resorcinol-aldehyde tetramer, named anoctol, the introduction of one bridge gives amono-bridged hexol, two bridges give anA,B orA,C-di-bridged tetrol, and three bridges give atri-bridged diol; the namecavitand refers to an octol rigidified by four bridges.
J. A. Tucker, C. B. Knobler, K. N. Trueblood, and D. J. Cram:J. Am. Chem. Soc. 111, 3688 (1989).
J. C. Sherman, C. B. Knobler, and D. J. Cram:J. Am. Chem. Soc. 113, 2194 (1991).
L. M. Tunstad, J. A. Tucker, E. Dalcanale, J. Weiser, J. A., Bryant, J. C. Sherman, J. C. Helgeson, C. B. Knobler, and D. J. Cram:J. Org. Chem. 54, 1305 (1989).
G. Germain, P. Main, and M. M. Woofson:Acta Crystallogr. A27, 368 (1971).
Structure Determination Package: B. A. Frenz and Associates, Inc., College Station TX and Enraf-Nonius, Delft (1983).
International Tables for X-Ray Crystallography, Vol. IV, Kynoch Press Birmingham, England (1974). Distr. Kluwer Academic Publishers, Dordrecht, the Netherlands.
D. J. Cram:Science 219, 1177 (1983).
J. A. Bryant, M. T. Blanda, M. Vincenti, and D. J. Cram:J. Am. Chem. Soc. 113, 2167 (1991).
D. J. Cram, H.-J. Choi, J. A. Bryant, and C. B. Knobler:J. Am. Chem. Soc. 114, 7748 (1992)
D. J. Cram, L. M. Tunstad, and C. B. Knobler:J. Org. Chem. 57, 528 (1992).
F. A. Carey and R. J. Sundberg:Advanced Organic Chemistry Part B, 3rd edition, Ch. 3., Plenum Press, New York (1990).
R. Kuhn and H. Trischmann:Liebigs Ann. Chem. 611, 117 (1958).
These tetraesters are used in the reaction with upper rim functionalized calix[4]arene derivatives, which will be published elsewhere.
W. D. S. Motherwell and W. Clegg:PLUTO, Program for plotting molecular and crystal structures, University of Cambridge, England (1978).
F. A. Carey and R. J. Sundberg:Advanced Organic Chemistry Part A., 3rd edition Ch. 10, Plenum Press, New York (1990).
A. I. Biggs and R. A. Robinson:J. Chem. Soc. 388 (1960).
W. E. Parham and R. M. Piccirilli:J. Org. Chem. 42, 257 (1977), and references therein.
The procedure for iodination is described elsewhere: P. Timmerman, W. Verboom D. N. Reinhoudt, A. Arduini, S. Grandi, A. R. Sicuri, A. Pochini, and R. Ungaro:Synthesis 185 (1994).
D. J. Gallagher and P. Beak:J. Am. Chem. Soc. 113, 7984 (1991), and references therein.
Author information
Authors and Affiliations
Additional information
This paper is dedicated to the commemorative issue on the 50th anniversary of calixarenes.
Supplementary Data. A list of observed and calculated structure factors have been deposited with the British Document Supply Centre as Supplementary Publication No. SUP 82170 (50 pages)
Rights and permissions
About this article
Cite this article
Timmerman, P., Boerrigter, H., Verboom, W. et al. Proximally functionalized cavitands and synthesis of a flexible hemicarcerand. J Incl Phenom Macrocycl Chem 19, 167–191 (1994). https://doi.org/10.1007/BF00708981
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00708981