Summary
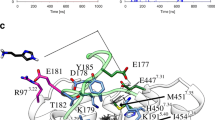
Mutation studies on the histamine H2 receptor were reported by Gantz et al. [J. Biol. Chem., 267 (1992) 20840], which indicate that both the mutation of the fifth transmembrane Asp186 (to Ala186) alone or in combination with Thr190 (to Ala190) maintained, albeit partially, the cAMP response to histamine. Recently, we have shown that histamine binds to the histamine H2 receptor as a monocation in its proximal tautomeric form, and, moreover, we suggested that a proton is donated from the receptor towards the tele-position of the agonist, thereby triggering the biological effect [Nederkoorn et al., J. Mol. Graph., 12 (1994) 242; Eriks et al., Mol. Pharmacol., 44 (1993) 886]. These findings result in a close resemblance with the catalytic triad (consisting of Ser, His and Asp) found in serine proteases. Thr190 resembles a triad's serine residue closely, and could also act as a proton donor. However, the mutation of Thr190 to Ala190 — the latter is unable to function as a proton donor — does not completely abolish the agonistic cAMP response. At the fifth transmembrane α-helix of the histamine H2 receptor near the extracellular surface, another amino acid is present, i.e. Tyr182, so an alternative couple of amino acids, Tyr182 and Asp186, could constitute the histamine binding site at the fifth α-helix instead of the (mutated) couple Asp186 and Thr190. In the first part of our present study, this hypothesis is investigated with the aid of an oligopeptide with an α-helical backbone, which represents a part of the fifth transmembrane helix. Both molecular mechanics and ab initio data lead to the conclusion that the Tyr182/Asp186 couple is most likely to act as the binding site for the imidazole ring present in histamine.
Similar content being viewed by others
References
Weinstein H., Mazurek A.P., Osman R. and Topiol S., Mol. Pharmacol., 29 (1986) 28.
Gantz I., DelValle J., Wang L., Tashiro T., Munzert G., Guo Y.-J., Konda Y. and Yamada T., J. Biol. Chem., 267 (1992) 20840.
Nederkoorn P.H.J., Vernooijs P., Donné-Op den Kelder G.M., Baerends E.J. and Timmerman H., J. Mol. Graph., 12 (1994) 242.
Eriks J.C., Van der Goot H. and Timmerman H., Mol. Pharmacol., 44 (1993) 886.
Nagy P.I., Durant G.J., Hoss W.P. and Smith D.A., J. Am. Chem. Soc., 116 (1994) 4898.
Carter P. and Wells J.A., Nature, 332 (1988) 564.
Warshel A., Naray-Szabo G., Sussman F. and Hwang J.-K., Biochemistry, 28 (1989) 3629.
Gantz I., Munzert G., Tashiro T., Schäffer M., Wang L., DelValle J. and Yamada T., Biochem. Biophys. Res. Commun., 178 (1991) 1386.
Zubay G., In Biochemistry, Wm. C. Brown Publishers, Dubuque, IA, U.S.A., 1993.
Bashford D. and Karplus M., Biochemistry, 29 (1990) 10219.
Chem-X Reference Guide, Chemical Design Ltd., Oxon, U.K., July 1994.
Darbey N.J. and Creighton T.E., In Rickwood D. (Ed.) Protein Structure, IRL Press, Oxford, U.K., 1993, pp. 1–22.
Oliveira L., Paiva A.M.C. and Vriend G., J. Comput.-Aided Mol. Design, 7 (1993) 649.
Némethy G. and Scheraga H.A., Rev. Biophys., 10 (1977) 239.
Vriend G. and Eijsink V., J. Comput.-Aided Mol. Design, 7 (1993) 367.
Cambridge Structural Database; Refcode hisahc 10: Bonnet J.J., Jeannin Y. and Laaouini M., Bull. Soc. Fr. Miner. Cri., 98 (1975) 208.
Ippolito J.A., Alexander R.S. and Christianson D.W., J. Biol. Chem., 215 (1990) 457.
Del Re G., Gavuzzo E., Giglio E., Lelj F., Mazza F. and Zappia V., Acta Crystallogr., B 33 (1977) 3289.
Van Duijneveldt-Van de Rijdt J.G.C.M. and Van Duijneveldt F.B., J. Am. Chem. Soc., 93 (1971) 5644.
Smit P.H., Derissen J.L. and Van Duijneveldt F.B., J. Chem. Phys., 67 (1977) 274.
GAMESS-UK is a package of ab initio programmes written by Guest, M.F., Van Lenthe, J.H., Kendrick, J., Schoeffel, K., Sherwood, P. and Harrison, R.J., with contributions from Amos, R.D., Buenker, R.J., Dupuis, M., Handy, N.C., Hillier, I.H., Knowles, P.J., Bonacic-Koutecky, V., Von Niessen, W., Saunders, V.R. and Stone, A.J. The package is derived from the original GAMESS code, see Ref. 22.
Dupuis, M., Spangler, D. and Wendoloski, J., GAMESS, Natural Resource of Computational Chemistry Software Catalog, Vol. 1, Program No. QG01, 1980.
Guest M.F., Fantucci P., Harrison R.J., Kendrick J., Van Lenthe J.H., Schoeffel K. and Sherwood P., GAMESS-UK User's Guide and Reference Manual, CFS Ltd., Daresbury Laboratory, Daresbury, U.K., 1993.
Van Lenthe J.H., Van Duijneveldt-Van de Rijdt J.G.C.M. and Van Duijneveldt F.B., In Lawley K.P. (Ed.) Ab Initio Methods in Quantum Chemistry, Wiley, New York, NY, U.S.A., 1987, pp. 521–565.
Kolos W., Theor. Chim. Acta, 54 (1980) 187.
Boys S.F. and Bernardi F., Mol. Phys., 19 (1970) 553.
Gutowski M., Van Duijneveldt-Van der Rijdt J.G.C.M., Van Lenthe J.H. and Van Duijneveldt F.B., J. Chem. Phys., 98 (1993) 4728.
Evans S.V., J. Mol. Graph., 11 (1993) 134.
Pyykkoe P. and Zhao Y., Report HUKI, 1 (1989) 89.
Sippl W., Stark H. and Höltje H.-D., Quant. Struct.-Act. Relatsh., 14 (1995) 121.
Marquart M., Walter J., Deisenhofer J., Bode W. and Huber R., Acta Crystallogr., B 39 (1983) 480.
Daggett V., Schröder S. and Kollman P., J. Am. Chem. Soc., 113 (1991) 8926.
Topiol S., Trends Biochem. Sci., 12 (1987) 419.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nederkoorn, P.H.J., van Lenthe, J.H., van der Goot, H. et al. The agonistic binding site at the histamine H2 receptor. I. Theoretical investigations of histamine binding to an oligopeptide mimicking a part of the fifth transmembrane α-helix. J Computer-Aided Mol Des 10, 461–478 (1996). https://doi.org/10.1007/BF00124476
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00124476