Abstract
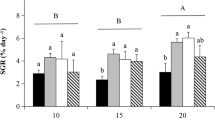
Acclimation responses of the red alga Gracilaria tenuistipitata var. liui collected on the northwest coast of Philippines were determined in laboratory setups and outdoor cultivation tanks in Haifa, Israel. Growth under laboratory conditions was influenced by all three variables studied, namely, temperature (20 or 30 °C), salinity (20, 30 or39‰) and seawater pH (6.5, 7.0, 8.0 or ≥ 9.0). In 250 mL flasks lacking pH control growth was influenced by temperature only at 20 ‰, whereas at 39 ‰, growth rates were similar at 20 or 30 °C. In 500 mL cylinders in which pH was controlled, growth rates were significantly different at a pH of 6.5 and 7.0 for all salinities, with maximal rates occurring in 39 ‰. At pH 8.0, and above, growth rates between salinities were similar and reduced to approximately 50% at a pH of 9.0 compared to rates at a pH of 6.5. Photosynthesis responses generally resembled growth responses both, in 250 mL and 500 mL cultures. In 40-L outdoor tanks, weekly growth and agar yields were apparently enhanced by increasing light intensities (up to full sunlight) and nutrient concentrations (up to 0.2 mM PO3 2- and 2.0 mM NH4 +), and rates averaged four times higher than rates determined in the smaller flask cultures. This study shows broad salinity tolerance of G. tenuistipitata var. liui and its ability to sustain growth rates that are among the highest measured for Gracilaria spp. in outdoor cultures.
Similar content being viewed by others
References
Beer S (1994) Mechanisms of inorganic carbon acquisition in marine macroalgae (with special reference to chlorophyta). In Chapman DJ, Round F (eds), Progress in Phycological Research, Biopress Ltd., Bristol, England: 179–207.
Beer S, Eshel A (1983) Photosynthesis of Ulva sp. II. Utilization of CO2 and HCO¯3 when submersed. J. exp. mar. Biol. Ecol. 70: 99–106.
Beer S, Israel A (1990) Photosynthesis of Ulva lactuca. IV. pH, carbonicanhydrase, and inorganic carbon conversions in the unstirred layer. Pl. Cell Environ. 13: 555–560.
Burkhardt S, Riebesell U (1997) CO2 availability affects elemental composition (C:N:P) of the marine diatom Skeletonema costatum. Mar. Ecol. Progr. Ser. 155: 67–76.
Chiang YM (1981) Cultivation of Gracilaria (Rhodophycophyta, Gigartinales) in Taiwan. In Levring T (ed.), Proceedings of Xth International Seaweed Symposium. Walter de Gruyter & Co., Berlin, New York, 569–574.
Craigie JS, Leigh C (1978) Carrageenans and agars. In Hellebust JA, Craigie JS (eds), Handbook of Phycological Methods. Cambridge University Press. Cambridge, 109–131.
Dawes CJ (1998) Marine Botany. Second edition. J. Wiley & Sons, New York. 480 pp.
Dawes CJ, Orduna-Rojas J, Robledo D (1999) Response of the tropical red seaweed Gracilaria cornea to temperature, salinity and irradiance. J. appl. Phycol. 10: 419–425.
Friedlander M (1991) Growth rate, epiphyte biomass and agar yield of Gracilaria conferta in an annual outdoor experiment. 1. Irradiance and nitrogen. Biores. Technol. 38: 203–208.
Friedlander M (1992) Gracilaria conferta and its epiphytes: the effect of culture conditions on growth. Bot. mar. 35: 423–428.
Friedlander M, Krom MD, Ben-Amotz A (1991) The effect of light and ammonium ongrowth, epiphytes and chemical constituents of Gracilaria conferta in outdoor cultures. Bot. mar. 34: 161–166.
Friedlander M, Levy I (1995) Cultivation of Gracilaria in outdoor tanks and ponds. J. appl. Phycol. 7: 315–324.
Frost-Christensen H, Sand-Jensen K (1990) Growth rate and carbon affinity of Ulva lactuca under controlled levels of carbon, pH and oxygen. Mar. Biol. 104: 497–501.
Haglund K, Pedersen M (1992) Growth of the red alga Gracilaria tenuistipitata at high pH. Influence of some environmental factors and correlation to an increased carbonic-anhydrase activity. Bot. mar. 35: 579–587.
Haglund K, Pedersen M(1993) Outdoor pond cultivation of the marine red alga Gracilaria tenuistipitata var. liui Zhang et Xia (Gracilariales, Rhodophyta) in brackish water in Sweden. Growth, nutrient uptake, cocultivation with rainbow trout and epiphyte control. J. appl. Phycol. 5: 271–284.
Israel A, Friedlander M (1998) Inorganic carbon utilisation and growth abilities in the marine red macroalga Gelidiopsis sp. Isr. J. Plant Sci. 46: 117–124.
Johnson KF (1982) Carbon dioxide hydration and dehydration kinetics in seawater. Limnol. Oceanogr. 27: 849–855.
Lapointe BE, Tenore KR, Dawes CJ (1984) Interaction between light and temperature on the physiological ecology of Gracilaria tikvahiae. 1. Growth, photosynthesis and respiration. Mar. Biol. 80: 161–170.
Levitt GJ, Bolton JJ (1990) Seasonal primary production of understory Rhodophyta in an upwelling system. J. Phycol. 26: 214–220.
Lowry OM, Rosebrough NT, Farr AL, Randall JR (1951) Protein measurement with folic phenol reagent. J. biol. Chem. 193: 263–275.
Maberly SC (1990) Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. J. Phycol. 26: 439–449.
Moon RE, Dawes CJ (1976) Pigment changes and photosynthetic rates under selected wavelengths in the growing tips of Eucheuma isiforme v. denudatum during vegetative growth. Br. phycol. J. 11: 165–175.
Moran R (1982) Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 69: 1376–1381.
Provasoli L (1968) Media and prospects for cultivation of marine algae. In Watanabe A, Gattori A (eds), Culture and Collection of Algae. Proc. U.S.-Japan Conf., Hakonte, Jap. Soc. Plant Physiol.: 63–75.
Sokal RR, Rohlf FJ (1998) Biometry. The principles and practice of statistics in biological research. Third edition. Freeman WH. New York. 887 pp.
Tseng CK, Xia B-M (1999) On the Gracilaria in theWestern Pacific and the Southeastern Asia region. Bot. mar. 42: 209–217.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Israel, A., Martinez-Goss, M. & Friedlander, M. Effect of salinity and pH on growth and agar yield of Gracilaria tenuistipitata var. liui in laboratory and outdoor cultivation. Journal of Applied Phycology 11, 543–549 (1999). https://doi.org/10.1023/A:1008141906299
Issue Date:
DOI: https://doi.org/10.1023/A:1008141906299