Abstract
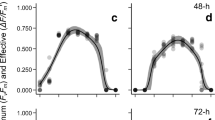
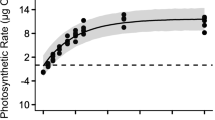
Photosynthesis-light response curves of Gelidium sesquipedale from the west coast of Portugal (Cape Espichel) were determined at four different depths, 3, 10, 15 and 22 m. Data acquisition using chlorophyll a fluorescence methodology and oxygen electrode measurements were compared. Response curves were determined over an increasing range of irradiance values (I), from darkness to 900 μmol photon m-2 s-1 PAR. In general, light response curves obtained for G. sesquipedale showed a similar pattern whether determined by the chlorophyll fluorescence method or by oxygen evolution. The photosynthetic capacity of G. sesquipedale decreased with depth, as expected, revealing a ‘sun’ and ‘shade’ acclimation pattern, between shallow and deeper waters.
Similar content being viewed by others
References
Abe S, Murakami A, Ohki K, Aruga Y, Fujita Y (1994) Changes in stoichiometry among PS I, PS II and cyt b6-f complexes in response to chromatic light for cell growth observed with the red alga Porphyra yezoensis. Plant Cell Physiol. 35: 901-906.
Beer S, Eshel A (1985) Determining phycoerythrin and phycocyanin concentrations in aqueous crude extract of red algae. Aust. J. mar. freshwat. Res. 36: 785-792.
Carmona R, Vergara JJ, Pérez-Lloréns JL, Figueroa FL, Niell FX (1996) Photosynthetic acclimation and biochemical responses of Gelidium sesquipedalecultured in chemostats under different qualities of light. Mar. Biol. 127: 25-34.
Conover WJ (1980) Practical Nonparametric Statistics. John Wiley & Sons, New York: 493 pp.
Coutinho R, Yoneshigue Y (1988) Diurnal variation in photosynthesis vs. irradiance curves from "sun' and "shade' plants of Pterocladia capillacea(Gmelin) Bornet et Thuret (Gelidiaciaceae: Rhodophyta) from Cabo Frio, Rio de Janeiro, Brazil. J. exp. mar. Biol. Ecol. 118: 217-228.
Duarte P, Ferreira JG (1995) Seasonal adaptation and short-term metabolic responses of Gelidium sesquipedaleto varying light and temperature. Mar. Ecol. Progr. Ser. 121: 289-300.
Figueroa FL (1996) Underwater light signals: Role of algal photoreceptors in the natural environment. Giorn. Bot. Ital. 130: 608-623.
Franklin LA, Forster RM (1997) The changing irradiance environment: consequences for marine macrophyte physiology, productivity and ecology. Eur. J. Phycol. 32: 207-232.
Genty B, Briantais J, Baker N (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990: 87-92.
Grossman AR, Schaefer MR, Chiang GG, Collier JL (1993) The phycobilisome, a light-harvesting complex responsive to environmental conditions. Microbiol. Rev. 57: 725-749.
Häder D-P, Figueroa FL (1997) Photoecophysiology of marine macroalgae. Photochem. Photobiol. 66: 1-14.
Hanelt D (1996) Photoinhibition of photosynthesis in marine macroalgae. In Figueroa F, Jiménez C, Pérez-Lloréns J, Niell FX (eds) Underwater Light and Algal Photobiology. Scientia Marina, Vol. 60(Suppl. 1), Barcelona: 243-248.
Hanelt D, Huppertz K, Nultsch W (1992) Photoinhibition of photosynthesis and its recovery in red algae. Bot. Acta 105: 278-284.
Hanelt D, Melchersmann B, Wiencke C, Nultsch W (1997) Effects of high light stress on photosynthesis of polar macroalgae in relation to depth distribution. Mar. Ecol. Progr. Ser. 149: 255-266.
Hanelt D, Nultsch W (1995) Field studies of photoinhibition show non-correlations between oxygen and fluorescence measurements in the Arctic red alga Palmaria palmata. J. Plant. Physiol. 145: 31-38.
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J. Phycol. 29: 729-739.
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 21: 540-547.
Kirk JTO (1994) Light and Photosynthesis in Aquatic Ecosystems, 2nd Edn. Cambridge University Press, Cambridge, UK. 509 pp.
Macler BA, Zupan JR (1991) Physiological basis for the cultivation of the Gelidiaceae. Hydrobiologia 221: 83-90.
Melo R (1998) Gelidiumcommercial exploitation: natural resources and cultivation. J. appl. Phycol. 10.
Ramus J (1981) The capture and transduction of light energy. In Lobban CS, Wynne MJ (eds) The Biology of the Seaweeds. Blackwell Scientific Publications, Oxford, UK: 786 pp.
Rowan S (1989) Photosynthetic Pigments of Algae, Cambridge University Press, Cambridge, UK.
Sagert S, Schubert H (1995) Acclimation of the photosynthetic apparatus of Palmaria palmata(Rhodophyta) to light qualities that preferentially excite photosystem I or photosystem II. J. Phycol. 31: 547-554.
Santos R (1993) A multivariate study of biotic and abiotic relationships in a subtidal algal stand. Mar. Ecol. Progr. Ser. 94: 181-190.
Santos R, Duarte P (1991) Marine plant harvest in Portugal. J. appl. Phycol. 3: 11-18.
Schreiber U, Bilger W, Neubauer C (1995) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivophotosynthesis. In Schulze E-D, Caldwell MM (eds) Ecophysiology of Photosynthesis, Springer-Verlag, Berlin: 49-70.
Seaton G, Walker D (1990) Chlorophyll fluorescence as a measure of photosynthetic carbon assimilation. Proc. R. Soc. Lond. B. 242: 29-35.
Serôdio J, Marques da Silva J, Catarino F (1997) Nondestructive tracing of migratory rhythms of intertidal benthic microalgae using in vivochlorophyll afluorescence. J. Phycol. 33: 542-553.
Strickland J, Parsons T (1968) A Practical Handbook of Seawater Analysis. Fish. Res. Bd Can., Ottawa: 167 pp.
Talling JF, Driver D (1963) Some problems in the estimation of chlorophyll in phytoplankton. Proceedings of Conference of Primary Productivities Measurements, Atomic Energy, Hawaii, USA. 7633: 142-166.
Torres M, Niell FX, Algarra P (1991) Photosynthesis of Gelidium sesquipedale: effects of temperature and light on pigment concentration, C/N ratio and cell-wall polysaccharides. Hydrobiologia 221: 77-82.
Torres M, Niell FX, Figueroa FL (1995) Photosynthetic metabolism and cell-wall polysaccharide accumulation in Gelidium sesquipedale(Clem.) Born. et Thur. under different light qualities. J. appl. Phycol. 7: 167-174.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Silva, J., Santos, R., Serôdio, J. et al. Light response curves for Gelidium sesquipedale from different depths, determined by two methods: O2 evolution and chlorophyll fluorescence. Journal of Applied Phycology 10, 295–301 (1998). https://doi.org/10.1023/A:1008057008447
Issue Date:
DOI: https://doi.org/10.1023/A:1008057008447