Abstract
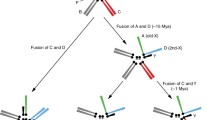
Y chromosome degeneration is characterized by structural changes in the chromosome architecture and expansion of genetic inertness along the Y chromosome. It is generally assumed that the heteromorphic sex chromosome pair has developed from a pair of homologues. Several models have been suggested. We use the unique situation of the secondary sex chromosome pair, neo-Y and neo-X (X2), in Drosophila miranda to analyze molecular mechanisms involved in the evolutionary processes of Y chromosome degeneration. Due to the fusion of one of the autosomes to the Y chromosome (about 2 Mya), a neo-Y chromosome and a neo-X chromosome, designated X2, were formed. Thus, formerly autosomal genes are inherited now on a pair of sex chromosomes in D. miranda. Analyzing DNA sequences from the X2 and neo-Y region, we observed a massive accumulation of DNA insertions on the neo-Y chromosome. From the analysis of several insertion elements, we present compelling evidence that the first step in Y chromosome degeneration is driven by the accumulation of transposable elements, especially retrotransposons. An enrichment of these elements along an evolving Y chromosome could account for the switch from a euchromatic into a heterochromatic chromatin structure.
Similar content being viewed by others
References
Barrio, E., A. Latorre, A. Moya & F.J. Ayala, 1992. Phylogenetic reconstruction of the Drosophila obscura group, on the basis of mitochondrial DNA. Mol. Biol. Evol. 9: 621-635.
Bone, R.J. & M.I. Kuroda, 1996. Dosage compensation regulatory proteins and the evolution of sex chromosomes in Drosophila. Genetics 144: 705-713.
Bull, J.J., 1983. Evolution of Sex Determining Mechanisms, pp.248- 269. Benjamin/ Cummings, Menlo Park, CA.
Charlesworth, B., 1978. A model for the evolution of Y chromosomes and dosage compensation. Proc. Natl. Acad. Sci. USA 75: 5618- 5622.
Charlesworth, D. & B. Charlesworth, 1979. The evolutionary genetics of sexual systems in flowering plants. Proc. R. soc. Lond. B 205: 513-530.
Charlesworth, D. & B. Charlesworth, 1980. Sex differences in fitness and selection for centric fusions between sex-chromosomes and autosomes. Genet. Res. 35: 205-214.
Charlesworth, B., 1991. The evolution of sex chromosomes. Science 251: 1030-1033.
Chalesworth, B., P. Sniegowski & W. Stephan, 1994. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 371: 215-220.
Charlesworth, B., 1996. The evolution of chromosomal sex determination and dosage compensation. Current Biology 6: 149-162.
Dobzhansky, Th., 1935. Drosophila miranda, a new species. Genetics 20: 377-391.
Ganguly, R., K.D. Swanson, K. Ray & R. Krishnan, 1992. A BamHI repeat element is predominantly associated with the degenerating neo-Y chromosome of Drosophila miranda but absent in the Drosophila melanogaster genome. Proc. Natl. Acad. Sci. USA 89: 1340-1344.
Goodfellow, P.N. & R. Lovell-Badge, 1993. SRY and sex determination in mammals. Annu. Rev. Genet. 27: 71-92.
Kraemer, C. & E.R. Schmidt, 1993. The sex-determining region of Chironomus thummi is associated with highly repetitive DNA and transposable elements. Chromosoma 102: 553-562.
Langley, C.H., E. Montgomery, R. Hudson, N. Kaplan & B. Charlesworth, 1988. On the role of unequal exchange in the containment of transposable element copy number. Genet. Res. 52: 223-235.
Lucchesi, J.C., 1978. Gene dosage compensation and the evolution of sex chromosomes. Science 202: 711-716.
Lucchesi, J.C., 1994. The evolution of heteromorphic sex chromosomes. BioEssays 16: 81-83.
Lucchesi, J. C., 1996. Dosage compensation in Drosophila and the ‘complex’ world of transcriptional regulation. BioEssays 18: 541-547.
MacKnight, R. H., 1939. The sex-determining mechanism of Drosophila miranda. Genetics 24: 180-201.
Marin, I., A. Franke, G. J. Bashaw & B. S. Baker, 1996. The dosage compensation system of Drosophila is co-opted by newly evolved X chromosomes. Nature 383: 160-163.
McClung, C.E., 1902. The accessory chromosome-sex determinant? Biol. Bull.3: 43-84.
Muller, H.J., 1918. Genetic variability, twin hybrids and constant hybrids, a case of balanced lethal factors. Genetics 3: 422-499.
Muller, H.J., 1932. Some genetic aspects of sex. Am. Nat. 66: 118- 138.
Muller, H.J., 1940. Bearings of the Drosophila work on systematics pp.185-268 in I. Huxley. The New Systematics edited by Oxford, Oxford University Press.
Nei, M., 1970. Accumulation of nonfunctional genes on sheltered chromosomes. Am. Nat. 104, 311-322.
Patterson, J.T. & W.S. Stone, 1952. Evolution in the genus Drosophila. New York, The Macmillan Company.
Pimpinelli, S., M. Berloco, L. Fanti, P. Dimitri, S. Bonaccorsi, E. Marchetti, R. Caizzi, C. Caggese & M. Gatti, 1995. Transposable elements are stable structural component of Drosophila melanogaster heterochromatin. Proc. Natl. Acad. Sci. USA 92: 3804-3808
Rice, W.R., 1987. Genetic hitchhiking and the evolution of reduced genetic activity of the Y sex chromosome. Genetics 116: 161-167.
Rice, W.R., 1994. Degeneration of a non-recombining chromosome. Science 263: 230-232.
Russo, C.A.M, N. Takezaki & M. Nei, 1995. Molecular phylogeny and divergence times of Drosophilid species. Mol. Biol. Evol. 12: 391-404.
Spradling, A.C., 1994. Transposable elements and the evolution of heterochromatin, pp. 69-83 in Molecular Evolution of Physiological Processes, edited by D. Fambrough. Rockefeller University Press, New York.
Steinemann, M., 1982. Multiple sex chromosomes in Drosophila miranda: A system to study the degeneration of a chromosome. Chromosoma 86: 59-76.
Steinemann, M., 1984. Telomere repeats within the neo-Y-chromosome of Drosophila miranda. Chromosoma 90: 1-5.
Steinemann, M. & S. Steinemann, 1990. Evolutionary changes in the organization of the major LCP gene cluster during sex chromosomal differentiation in the sibling species Drosophila persimilis, D. pseudoobscura and D. miranda. Chromosoma 99: 424-431.
Steinemann, M. & S. Steinemann, 1991. Preferential Y chromosomal location of TRIM, a novel transposable element of Drosophila miranda, obscura group. Chromosoma 101: 169-179.
Steinemann, M. & S. Steinemann, 1992. Degenerating Y chromosome of Drosophila miranda: A trap for retrotransposons. Proc. Natl. Acad. Sci. USA 89: 7591-7595.
Steinemann, M., S. Steinemann & F. Lottspeich, 1993. How Y chromosomes become genetically inert. Proc. Natl. Acad. Sci. USA 90: 5737-5741.
Steinemann, M. & S. Steinemann, 1993. A duplication including the Y allele of Lcp2 and the TRIM retrotransposon at the Lcp locus on the degenerating neo-Y chromosome of Drosophila miranda: Molecular structure and mechanisms by which it may have arisen. Genetics 134: 497-505.
Steinemann, M., S. Steinemann & B. M. Turner, 1996. Evolution of dosage compensation. Chromosome Research 4: 185-190.
Steinemann, M. & S. Steinemann, 1997. The enigma of Y chromosome degeneration: TRAM, a novel retrotransposon is preferentially located on the neo-Y chromosome of Drosophila miranda. Genetics 145: 261-266.
Stevens, N.M., 1905. Studies in spermatogenesis with especial reference to the ‘accessory chromosome’. Carn. Inst. Wash. Publ. Washington, D.C. 36: 3-32.
White, M.J.D., 1973. Sex chromosomes, pp. 129-146 in The Chromosomes, 6th edition. Chapman and Hall, London.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Steinemann, M., Steinemann, S. Enigma of Y chromosome degeneration: Neo-Y and Neo-X chromosomes of Drosophila miranda a model for sex chromosome evolution. Genetica 102, 409–420 (1998). https://doi.org/10.1023/A:1017058119760
Issue Date:
DOI: https://doi.org/10.1023/A:1017058119760