Abstract
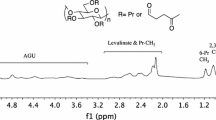
Two homologous series of regio- selectively sub stituted cellulose: 3-O-benzyl-2, 6-di-O-pivaloyl derivatives (Series 1) and 2,3,6-tri-O-acetyl derivatives (Series 2) up to an eicosamer (DP = 20), were synthesized for the first time by elongation of the carbohydrate chain from cellooctaose derivative 5 and subsequent deprotection. Some changing properties of the homologous series with increasing DP were examined. Gel permeation chromatography (GPC) analysis for series 1 and 2 indicated that plots of log M vs retention time gave a straight line. The plots of [M]n/n vs (n − 1)/n (where [M]n is the molecular rotation for an oligomer with DP = n) up to a hexadecamer 9 in series 2 gave straight lines, although the result for the eicosamer 11 deviated somewhat from the line
Similar content being viewed by others
REFERENCES
Baptistella, L. H. B., dos Santos, J. F., Ballabio, K. C. and Marsaioli, A. J. (1989) 1,8-Diazabicyclo [5.4.0] undec-7-ene as a Mild Deprotective Reagent for Acetyl Group. Synthesis 21, 436–438.
Buchanan, C. M., Hyatt, J. A. and Lowman, D. W. (1987) Two-Dimensional NMR of Polysaccharides: Spectral Assignment of Cellulose Triesters. Macromolecules 20, 2750–2754.
Buchanan, C. M., Hyatt, J. A., Kelly, S. S. and Little, J. L. (1990) á-D-Cellooligosaccharide Acetate: Physical and Spectroscopic Characterization and Evaluation as Models for Cellulose Triacetate. Macromolecules 23, 3747–3755.
Dickey, E. E. and Wolfrom, M. L. (1949) A Polymer-homologous Series of Sugar Acetates from the Acetolysis of Cellulose. J. Am. Chem. Soc. 71, 825–828.
Freudenberg, K. (1933) Tannin, Cellulose, Lignin.Berlin: Springer.
Gagnaire, D., Mancier, D. and Vincendon, M. (1980) Cellulose Organic Solution: A Nuclear Magnetic Resonance Investigation J. Polym. Sci., Polym. Chem. Ed. 18, 13–25.
Gast, J. G., Atalla, R. H. and McKelvey, R. D. (1980) The 13C-N.M.R. Spectra of the Xylo-and Cello-Oligosaccharide Carbohydr. Res. 84, 137–146.
Gessler, K., Krauss, N., Steiner, T., Betzel, C., Sandmann, C. and Saenger, W. (1994) Crystal Structure of â-D-Cellotetraose Hemihydrate with Implications for the Structure of Cellulose II: Science 266, 1027–1029.
Henrissat, B., Serge, P., Ivaroska, I., Winter, W. T. (1987) The Structure of Cellulose. In Multidisciplinary Approaches to the Structures of Model Compounds for Cellulose II. ACS Symp Ser 340, (R. H. Atalla, ed.). Washington DC: American Chemical Society, pp. 38–67.
Isogai, A. and Usuda, M. (1991) Preparation of Low-Molecular Weight Celluloses Using Phosphoric Acid. Mokuzai-Gakkaishi 37, 339–344.
Johnson, J. F. (1985) Size-exclusion (Gel-permeation) Chromatography. In Encyclopedia of Polymer Science and Engineering, Vol. 3 (H. F. Mark, N. M. Bikales, C. G. Overberger, and G. Menger eds). New York: Wiley-Interscience, pp. 501–523.
Kamitakahara, H., Nakatsubo, F. (1996) Substituent Effect on Ring-Opening Polymerization of Regioselectively Acylated 1,4-Anhydro-alpha-D-glucopyranose Derivatives. Macromolecules 29, 1119–1122.
Kamitakahara, H., Nakatsubo, F., Murakami, K. (1994) Synthesis of 1,4-Anhydro-á-D-Glucopyranose Derivative Havibg Acyl Groups. Mokuzai-Gakkaishi 40, 302–307.
Kamitakahara, H., Nakatsubo, F., Murakami, K. (1994b) Ring-Opening Polymerization of 1,4-Anhydro-á-D-glucopyranose Derivatives Having Acyl Groups and Synthesis of (1! 5)-â-DGlucofuranan. Macromolecules 27, 5937–5942.
Kamitakahara, H., Hori, M. and Nakatsubo, F. (1996) Substituent Effect on Ring-Opening Polymerization of Regioselectively Acylated á-D-Glucopyranose 1,2,4-Orthopivalate Derivatives. Macromolecules 29, 6126–6131.
Kawada, T., Nakatsubo, F. and Murakami, K. (1989a) Synthetic Studies of Cellulose. III. Synthesis of allyl 2,3,6-tri-O-4-O-( p-methoxybenzyl)-â-D-glucopyranoside and its conversion into glycosyl acceptor and glycosyl donor. Mokuzai Gakkaishi 35, 14–20.
Kawada, T., Nakatsubo, F. and Murakami, K. (1989b) Synthetic Studies of Cellulose. IV. Preparation of perbenzylated allyl 1,4-O-( p-methoxybenzyl) cellobioside by the imidate method. Mokuzai Gakkaishi 35, 21–25.
Kawada, T., Nakatsubo, F. and Murakami, K. (1990) Synthetic Studies of Cellulose VII. Synthesis of a Series of Perbenzylated Allyl 4n9-p-Methoxybenzyl Cellooligosaccharide Up to Octamer. Cellulose Chem. Technol. 24, 343–350.
Kawada, T., Nakatsubo, F. and Murakami, K. (1991) Synthetic Studies of Cellulose. IX. Reactivity and cleavage of three kinds of protective groups of synthesized cello-oligosaccharide derivatives. Mokuzai Gakkaishi 37, 930–934.
Kawada, T., Nakatsubo, F., Murakami, K. and Sakuno, T. (1994) Synthetic Studies of Cellulose XII. First chemical synthesis of cellooctaose acetate. Mokuzai Gakkaishi 41, 738–743.
Kobayashi, S., Kashiwa, K., Kawasaki, K. and Shoda, S. (1991) Novel method for polysaccharide synthesis using an enzyme: the first in vitro synthesis of celulose via a nonbiosynthetic path utilizing cellulaze as catalyst. J. Am. Chem. Soc. 113, 3079–3084.
Kunz, H. and Harreus, A. (1982) Glycosidsynthese mit 2,3,4,6-tetra-O-pivaloyl-á-Dglucopyranosylbromide. Liebigs. Ann. Chem. 41–48.
Mimura, M., Kitamura, S., Gotoh, S., Takeo, K., Urakawa, H. and Kajiwara, K. (1996) Conformation of cyclic and linear (1! 2)-â-D-glucans in aqueous solution. Carbohyd. Res. 289, 25–37.
Mizuno, T. (1968) Homologous Oligosaccharide. Denpun Tou Gikenho 37, 66–79.
Nakatsubo, F., Takano, T., Kawada, T., Someya, H., Harada, T., Shiraki, H. and Murakami, K. (1985) Synthetic Studies of Cellulose I, Synthetic design and selection of the protective groups: Memoirs of the College of Agriculture, Kyoto University 127, 37–47.
Nakatsubo, F., Takano, T., Kawada, T. and Murakami, K. (1989) Toward the Synthesis of Cellulose. Synthesis of Cellooligosaccharides. In Cellulose, Structural and Functional Aspects (J. F. Kennedy, G. O. Phillips, P. A. Williams, eds). London: Ellis Horwood, pp. 201–206.
Nakatsubo, F., Kamitakahara, H. and Hori, M. (1996) Cationic Ring-Opening Polymerization of 3,6-Di-O-benzyl-á-D-glucose 1,2,4-Orthopivalate and the First Chemical Synthesis of Cellulose: J. Am. Chem. Soc. 118, 1677–1681.
Nishimura, T., Nakatsubo, F. and Murakami, K. (1994) Synthetic studies of cellulose XI. High yield synthesis of cellotetraose derivative by convergent synthetic method. Mokuzai Gakkaishi 40, 44–49.
Nishimura, T. and Nakatsubo, F. (1996a) First synthesis of cellooctaose by a convergent synthetic method. Carbohydrate Research 294, 53–64.
Nishimura, T. and Nakatsubo, F. (1996b) First stepwise synthesis of cellulose analogs: Tetrahedron Lett. 37, 9215–9218.
Nishimura, T. and Nakatsubo, F. (1996c) Synthetic Studies of Cellulose: Selection of Protective Groups and First Stepwise Synthesis of Cellulose Derivatives: Abstracts of XVIII International carbohydrate symposium, July 21-26 Milano, Abstract. BP183. International Carbohydrate Organisation.
Nishimura, T., Takano, T., Nakatsubo, F. and Murakami, K. (1993) Synthetic studies of cellulose X. Selection of suitable starting materials for convergent synthesis of cello-oligosaccharide. Mokuzai Gakkaishi 39, 40–47.
Okuyama, K. and Noguchi, K. (1994) Structural Studies on Polysaccharides by using Structural Information of the Related Oligosaccharides. Kobunshi 43, 848–858.
Ohno, Y. (1977) The synthesis of cellulose by the method of organic chemistry. Sen-I Gakkaishi 33, 87–94.
Paulsen, H. (1982) Advances in Selective Chemical Synthesis of Complex Oligosaccharide. Angewandte Chemie (International Edition in English) 21, 155–173.
Raymond, S., Heyraud, A., Tarn Qui, D., Kvick, A. H. and Chanzy, H. (1995) Crystal and Molecular Structure of â-D-Cellotetraose Hemihydrate as a Model of Cellulose II. Macromolecules 28, 2096–2100.
Schlubach, H. M. and Luhrs, L. (1941) Ein Wirkung von Chlorowasserstoff auf Glucose, Synthese eines Polyglucan. Liebigs Ann. Chem. 547, 73–85.
Schmidt, R. R. (1986) New Methods for the Synthesis of Glycosides and Oligosaccharides-Are there Alternatives to the Koenigs-Knorr Methods? Angewandte Chemie (International Edition in English) 25, 212–235.
Schmidt, R. R. and Michel, J. (1982) Synthesis of linear and branched cellotetraose. Angewandte Chemie (International Edition in English) 21, 72–73.
Segal, L. (1966) Fractionation of cellulose trinitrates by gel permiation chromatography. J. Polym. Sci. 134B, 1011–1018.
Sinay¨, P. (1978) Recent advances in Glycosylation Reactions. Pure Appl. Chem. 50, 1437–1452.
Takano, T., Harada, Y., Nakatsubo, F. and Murakami, K. (1990a) Synthetic Studies of Cellulose VI, Effect of the substituent groups of the glycon on â-glycosylation. Cellulose Chem. Technol. 24, 333–341.
Takemoto, K. (1976) Trial to synthesize cellulosic macromolecules. Sen-I Gakkaishi 32, 101–106.
Takeo, K., Okushio, K., Fukuyama, K. and Kuge, T. (1983) Synthesis of cellobiose, cellotriose, cellotetraose and lactose. Carbohydr. Res. 121, 163–173.
Takano, T., Nakatsubo, F. and Murakami, K. (1987) Synthetic Studies of Cellulose. II. Synthesis of 1,4-substituted 2,3,6-tri-O-acetyl-â-D-Glucopyranoside. Mokuzai Gakkaishi 33, 667–675.
Takano, T., Nakatsubo, F. and Murakami, K. (1988) Synthetic Studies of Cellulose V, Effect of the 3-O-and 6-O-substituent groups of the aglycon on â-glycosylation. Cellulose Chem. Technol. 22, 135–145.
Takano, T., Harada, Y., Nakatsubo, F. and Murakami, K. (1990a) Synthetic Studies of Cellulose VI, Effect of the substituent groups of the glycon on â-glycosylation. Cellulose Chem. Technol. 24, 333–341.
Takano, T., Harada, Y., Nakatsubo, F. and Murakami, K. (1990b) Synthetic Studies of Cellulose VIII, Effect of the 3-O-substituent group of the aglycon on â-glycosylation (2). Mokuzai Gakkaishi 36, 212–217.
Takano, T., Nakatsubo, F. and Murakami, K. (1990c) A facile â-allylglycosylation in the presence of benzyl protective group using boron trifluoride etherate. Carbonhydr. Res. 203, 141–142.
Velluz, L., Valls, J. and Mathieu, J. (1967) Spatial arrangement and preparative organic synthesis. Angewandte Chemie(International Edition in English) 6, 778–789.
Wolfrom, M. L. and Dacon, J. C. (1952) The Polymer-homologous Series of Oligosaccharides from Cellulose. J. Am. Chem. Soc. 74, 5331–5333.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nishimura, T., Nakatsubo, F. Chemical synthesis of cellulose derivatives by a convergent synthetic method and several of their properties. Cellulose 4, 109–130 (1997). https://doi.org/10.1023/A:1018423503762
Issue Date:
DOI: https://doi.org/10.1023/A:1018423503762