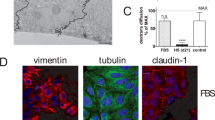
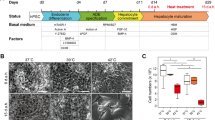
Many of the differentiated functions of hepatocytes are lost in culture, yet addition of certain medium supplements can aid in the retention of differentiated character. Therefore, the effect of time in monolayer culture on rat hepatocyte glutathione (GSH) synthesis and sensitivity to the GSH detoxicated xenobiotic ethacrynic acid was examined in cultures with and without medium supplementation by transferrin and sodium selenite. GSH content was found to be about 12 nmol/µg DNA at 4 hr in culture and to approximately triple by 24 hr. Intracellular GSH levels continued to increase in transferrin/sodium selenite-supplemented cultures, from 32 to 41.6 nmol/µg DNA, while GSH levels in unsupplemented cultures declined to 18 nmol/µg DNA. However, the rate of GSH synthesis after diethylmaleate depletion was found to decrease from 4.2 to 2.8 nmol/hr/µg DNA at 4 and 24 hr after inoculation, respectively. GSH repletion rate increased to 3.9 nmol/hr/µg DNA at 48 hr. The GSH accumulation rate after depletion in supplemented cultures did not vary significantly over the initial 48 hr. Incubation for 3 hr with 100 µM ethacrynic acid (EA) did not elicit an increase in LDH leakage in hepatocyte monolayers after 4 or 48 hr in culture or in cultures with supplemented medium at any time point tested. Cultures 24 hr in medium without transferrin/sodium selenite supplementation exhibited significant LDH leakage after 3 hr of EA treatment. Over the 3 hr EA treatment, intracellular GSH content was decreased in all cultures. Only in the 24 hr unsupplemented cultures did GSH depletion exceed the 90% level previously associated with depletion of the mitochondrial pool of GSH and EA toxicity in hepatocytes. The experiments show that during the redifferentiation of hepatocytes in culture, a transient period occurs when apparent GSH synthesis is depressed and enhanced sensitivity to GSH-detoxicated compounds is observed. This period of increased sensitivity is prevented or at least delayed by inclusion of supplemental transferrin and sodium selenite, suggesting that redifferentiation can be regulated by extracellular influences.
Similar content being viewed by others
Abbreviations
- CYSSG:
-
cysteine-glutathione mixed disulfide
- DEM:
-
diethyl maleate
- EA:
-
ethacrynic acid
- GSH:
-
reduced glutathione
- GSSG:
-
oxidized glutathione
- HBS:
-
HEPES buffered saline
- HWME:
-
hepatocyte Williams' Medium E (WME with insulin, corticosterone and 0.5 mM methionine)
- LDH:
-
lactate dehydrogenase
- TS-HWME:
-
transferrin/sodium selenite-supplemented HWME
- WME:
-
Williams' Medium E
References
ARKIN, H. and COLTON, R.R. (1970). Statistical Methods, 5th Edition, pp. 153–157. Barnes and Noble, Inc., New York, New York.
BARNES, D. and SATO, G. (1980a). Serum-free cell culture: A unifying approach. Cell 22:649–653.
BARNES, D. and SATO, G. (1980b). Methods for growth of cultured cells in serum-free medium. Anal. Biochem. 102:255–270.
BEATTY, P.W. and REED, D.J. (1980). Involvement of the cystathionine pathway in the biosynthesis of glutathione by isolated rat hepatocytes. Arch. Biochem. Biophys. 204: 80–87.
BOREK, C. and WILLIAMS, G.M. (Eds.) (1980). Cellular systems for toxicity testing. Ann. N.Y. Acad. Sci. 349:1–429.
BURK, R.F. (1983). Biological activity of selenium. Ann. Rev. Nutr. 3:53–70.
CROCI, T. and WILLIAMS, G.M. (1985). Activities of several phase I and phase II xenobiotic biotransformation enzymes in cultured hepatocytes from male and female rats. Biochem. Pharmacol. 34:3029–3035.
ERWIN, B.G., STOSCHECK, C.M. and FLORINI, L.R. (1981). A rapid fluorometric method for the estimation of DNA in cultured cells. Anal. Biochem. 110:291–294.
FARISS, M.W. and REED, D.J. (1983). Measurement of glutathione and glutathione disulfide efflux from isolated rat hepatocytes. In: Isolation, Characterization, and Use of Hepatocytes (R.A. Harris and N.W. Cornell, eds), pp. 349–355. Elsevier-North Holland Biochemical Press, Amsterdam.
GUENGERICH, F.P. (1984). Factors involved in the regulation of the levels and activities of rat liver cytochromes. Biochem. Soc. Trans. 2:68–70.
HARMON, A.W. and FISHER, L.J. (1983). Hamster hepatocytes in culture as a model for acetaminophen toxicity: Studies with inhibitors of drug metabolism. Toxicol. Appl. Pharmacol. 71:330–341.
HOGBERG, J. and KRISTOFSEN, A. (1977). A correlation between glutathione levels and cellular damage in isolated hepatocytes. Eur. J. Biochem. 74:77–82.
KLETZIEN, R.F., STUMPO, D.J., KELLEY, D.S. and TEDDERUD, C.G. (1983). Primary cultures of hepatocytes as a model system for studies on the chronic effects of hormones on hepatic carbohydrate metabolism. In: Isolation, Characterization, and Use of Hepatocytes (R.A. Harris and N.W. Cornell, eds.), pp. 77–86. Elsevier-North Holland Biochemical Press, Amsterdam.
KLETZIEN, R.F., WEBER, C.A., BUTCHER, F.R. and STUMPO, D.J. (1982). Glucagon and choleragen stimulation of glycogenolysis in primary cultures of adult rat liver parenchymal cells: Lack of involvement of the glucocorticoids. J. Cell Physiol. 110:304–310.
LAISHES, B.A. and WILLIAMS, G.M. (1976). Conditions affecting primary cultures of functional rat hepatocytes. II. Dexamethasone-enhanced longevity and maintenance of morphology. In Vitro 12:821–832.
MENDOZA-FIGUEROA, T. (1984). Dimethylnitrosamine genotoxicity in rat liver primary cell cultures with low cytochrome P-450 levels. J. Appl. Toxicol. 4:297–303.
MEREDITH, M.J. and REED, D.J. (1982). Status of the mitochrondrial pool of glutathione in the isolated hepatocyte. J. Biol. Chem. 257:3747–3753.
MITCHELL, D.A., ACOSTA, D. and BRUCKNER, J.V. (1985). Role of glutathione depletion in the cytotoxicity of acetaminophen in a primary culture system of rat hepatocytes. Toxicology 37:127–146.
NAKABAYASHI, N., TAKETA, K., MIYANO, K., TAMANE, T. and SATO, J. (1982). Growth of human hepatoma cell lines with differentiated functions in chemically defined medium. Cancer Res. 42:3858–3863.
NEWMAN, S. and GUZELIAN, P. (1982). Stimulation of de novo synthesis of cytochrome P-450 by phenobarbital in primary nonproliferating cultures of adult rat hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 79:2922–2926.
REED, D.J., BABSON, J.R., BEATTY, P.W., BRODIE, A.E., ELLIS, W.W. and POTTER, D.W. (1980). High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Analyt. Biochem. 106:55–62.
REED, D.J. and BEATTY, P. W. (1978). The role of the cystathionine pathway in glutathione regulation by isolated hepatocytes. In: Functions of Glutathione in the Liver and Kidney (H. Seis and A. Wendel, eds.), p. 13. Springer-Verlag, Berlin.
REED, D.J. and ORRENIUS, S. (1977). The role of methionine in glutathione biosynthesis by isolated hepatocytes. Biochem. Biophys. Res. Commun. 77:1257–1264.
RICHMAN, P. and MEISTER, A. (1975). Regulation of γ-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J. Biol. Chem. 250:1422–1426.
ROSE, W.C. and WIXOM, R.L. (1955). The amino acid requirements of man. XIV. The sparing effect of tyrosine on the phenylalanine requirement. J. Biol. Chem. 217:95–106.
STEWARD, R.A., DANNAN, G.A., GUZELIAN, P.S. and GUENGERICH, F.P. (1985). Changes in the concentration of seven forms of cytochrome P-450 in primary cultures of adult rat hepatocytes. Mol. Pharmacol. 27:125–132.
TAMURA, J., OHKUMA, S., IDA, S., ZUO, P.P. and KURIYAMA, K. (1984). Cysteine uptake and taurine biosynthesis in freshly isolated and primary cultured rat hepatocytes. Cell Biochem. Function 2:195–200.
VINA, J., HEMS, R. and KREBS, H.A. (1978). Maintenance of glutathione content in isolated hepatocytes. Biochem. J. 170:627–630.
WACKER, W.E.C., ULMER, D.D. and VALLEE, B.L. (1956). Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N. Engl. J. Med. 255:449–453.
WEBER, C.A. and KLETZIEN, R.F. (1982). Hormonal and nutritional factors influencing glycogen deposition in primary cultures of rat liver parenchymal cells. J. Cell Physiol. 110:300–303.
WILLIAMS, G. M. and GUNN, J. M. (1974). Long-term cell culture of adult rat liver epithelial cells. Exp. Cell Res. 89:139–142.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meredith, M.J. Culture duration alters the glutathione content and sensitivity to ethacrynic acid of rat hepatocyte monolayer cultures. Cell Biol Toxicol 2, 495–505 (1986). https://doi.org/10.1007/BF00117851
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00117851