Abstract
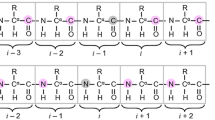
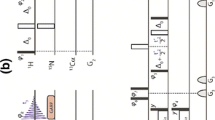
A combination of three heteronuclear three-dimensional NMR experiments tailored for sequential resonance assignments in uniformly 15N, 13C-labeled flexible polypeptide chains is described. The 3D (H)N(CO-TOCSY)NH, 3D (H)CA(CO-TOCSY)NH and 3D (H)CBCA(CO-TOCSY)NH schemes make use of the favorable 15N chemical shift dispersion in unfolded polypeptides, exploit the slow transverse 15N relaxation rates of unfolded polypeptides in high resolution constant-time [1H, 15N]-correlation experiments, and use carbonyl carbon homonuclear isotropic mixing to transfer magnetization sequentially along the amino acid sequence. Practical applications are demonstrated with the 100-residue flexible tail of the recombinant human prion protein, making use of spectral resolution up to 0.6 Hz in the 15N dimension, simultaneous correlation with the two adjacent amino acid residues to overcome problems associated with spectral overlap, and the potential of the presently described experiments to establish nearest-neighbor correlations across proline residues in the amino acid sequence.
Similar content being viewed by others
References
Abragam, A. (1961) Principles of Nuclear Magnetism, Oxford University Press, New York, NY, pp. 305ff.
Bartels, Ch., Xia, T., Billeter, M., Güntert, P. and Wüthrich, K. (1995) J. Biomol. NMR, 6, 1–10.
Bax, A. and Grzesiek, S. (1993) Acc. Chem. Res., 26, 131–138.
Bax, A. and Ikura, M. (1991) J. Biomol. NMR, 1, 99–104.
Bodenhausen, G. and Ruben, D. (1980) Chem. Phys. Lett., 69, 185–188.
Bracken, C., Palmer, A.G. III and Cavanagh, J. (1997) J. Biomol. NMR, 9, 94–100.
Braun, D., Wider, G. and Wüthrich, K. (1994) J. Am. Chem. Soc., 116, 8466–8469.
Braunschweiler, L. and Ernst, R.R. (1983) J. Magn. Reson., 53, 521–528.
Bundi, A. and Wüthrich, K. (1979) Biopolymers, 18, 299–311.
Cavanagh, J., Palmer, A.G. III, Wright, P.E. and Rance, M. (1991) J. Magn. Reson., 91, 429–436.
Donne, D.G., Viles, J.H., Groth, D., Mehlhorn, I., James, T.L., Cohen, F.E., Prusiner, S.B., Wright, P.E. and Dyson, H.J. (1997) Proc. Natl. Acad. Sci. USA, 94, 13452–13457.
Dyson, H.J. and Wright, P.E. (1998) Nat. Struct. Biol., 5, 499–503.
Grzesiek, S. and Bax, A. (1992) J. Am. Chem. Soc., 114, 6291–6293.
Grzesiek, S. and Bax, A. (1993) J. Am. Chem. Soc., 115, 12593–12594.
Grzesiek, S. and Bax, A. (1997) J. Biomol. NMR, 9, 207–211.
Grzesiek, S., Anglister, J. and Bax, A. (1993a) J. Magn. Reson., B 101, 114–119.
Grzesiek, S., Anglister, J., Ren, H. and Bax, A. (1993b) J. Am. Chem. Soc., 115, 4369–4370.
Güntert, P., Dötsch, V., Wider, G. and Wüthrich, K. (1992) J. Biomol. NMR, 2, 619–629.
Kay, L.E., Keifer, P. and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663–10665.
Kay, L.E., Ikura, M., Tschudin, R. and Bax, A. (1990) J. Magn. Reson., 89, 496–514.
Kupče, E. and Freeman, R. (1995) J. Magn. Reson., A 115, 273–276.
Liu, A. (1999) NMR Spectroscopy with Prion Proteins and Prion Protein Fragments, Ph.D. Thesis No. 13234, ETH Zürich.
Logan, T.M., Olejniczak, E.T., Xu, R.X. and Fesik, S.W. (1993) J. Biomol. NMR, 3, 225–231.
Marion, D., Ikura, M., Tschudin, R. and Bax, A. (1989) J. Magn. Reson., 85, 393–399.
Matsuo, H., Kupce, E., Li, H. and Wagner, G. (1996) J. Magn. Reson., B 111, 194–198.
McCoy, M. and Mueller, L. (1992) J. Am. Chem. Soc., 114, 2108–2112.
McCoy, M. and Mueller, L. (1993) J. Magn. Reson., A 101, 122–130.
Morris, G.A. and Freeman, R. (1979) J. Am. Chem. Soc., 101, 760–762.
Muhandiram, D.R. and Kay, L.E. (1993) J. Magn. Reson., B 103, 203–216.
Rance, M. (1987) J. Magn. Reson., 74, 557–564.
Riek, R., Hornemann, S., Wider, G., Glockshuber, R. and Wüthrich, K. (1997) FEBS Lett., 413, 282–288.
Santoro, J. and King, G.C. (1992) J. Magn. Reson., 97, 202–207.
Schätzl, H.M., Da Costa, M., Taylor, L., Cohen, F.E. and Prusiner, S.B. (1995) J. Mol. Biol., 245, 362–374.
Shaka, A.J., Barker, P.B. and Freeman, R. (1985) J. Magn. Reson., 64, 547–552.
Shaka, A.J., Lee, C.J. and Pines, A. (1988) J. Magn. Reson., 77, 274–293.
Shaka, A.J., Keeler, J., Frenkiel, T. and Freeman, R. (1983) J. Magn. Reson., 52, 335–338.
SØ rensen, O.W., Rance, M. and Ernst, R.R. (1984) J. Magn. Reson., 56, 527–534.
Tycko, R., Pines, A. and Gluckenheimer, R. (1985) J. Chem. Phys., 83, 2775–2802.
Vuister, G.W. and Bax, A. (1992) J. Magn. Reson., 98, 428–435.
Wang, A.C., Grzesiek, S., Tschudin, R., Lodi, P.J. and Bax, A. (1995) J. Biomol. NMR, 5, 376–382.
Wider, G. (1998) Prog. NMR Spectrosc., 32, 193–275.
Wittekind, M. and Müller, L. (1993) J. Magn. Reson., B 101, 201–205.
Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids, Wiley, New York, NY.
Wüthrich, K. (1994) Curr. Opin. Struct. Biol., 4, 93–99.
Zahn, R., von Schroetter, C. and Wüthrich, K. (1997) FEBS Lett., 417, 400–404.
Zahn, R., Liu, A., Lührs, T., Riek, R., von Schrötter, C., Lopez-Garcia, F., Billeter, M., Calzolai, L., Wider, G. and Wüthrich, K. (2000) Proc. Natl. Acad. Sci. USA, 97, 145–150.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, A., Riek, R., Wider, G. et al. NMR experiments for resonance assignments of 13C, 15N doubly-labeled flexible polypeptides: Application to the human prion protein hPrP(23–230). J Biomol NMR 16, 127–138 (2000). https://doi.org/10.1023/A:1008305022907
Issue Date:
DOI: https://doi.org/10.1023/A:1008305022907