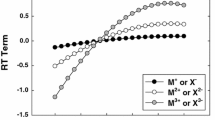
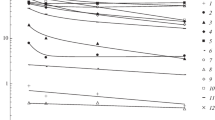
Abstract
This paper reviews the present status of the Pitzer chemical equilibrium model, which can be used to characterize the one-atmosphere activity coefficients of ionic and non-ionic solutes in natural waters as a function of temperature and ionic strength. The model considers the ionic interactions of the major seasalt ions (H, Na, K, Mg, Ca, Sr, Cl, Br, OH, HCO3, B(OH)4, HSO4, SO4, CO3, CO2, B(OH)3, H2O) and is based on the 25 °C model of Weare and co-workers. The model has been extended by a number of workers so that reasonable estimates can be made of the activity coefficients of most of the major seasalt ions from 0 to 250 °C. Recently coefficients for a number of solutes that are needed to determine the dissociation constants of the acids from 0 to 50 °C (H3CO3, B(OH)3, H2O, HF, HSO -4 , H3PO4, H2S, NH +4 etc.) have been added to the model. These results have been used to examine the carbonate system in natural waters and determine the activity of inorganic anions that can complex trace metals. The activity and osmotic coefficients determined from the model are shown to be in good agreement with measured values in seawater. This model can serve as the foundation for future expansions that can examine the activity coefficient and speciation of trace metals in natural waters. At present this is only possible from 0 to 50 °C over a limited range of ionic strengths (<1.0) due to the limited stability constants for the formation of the metal complexes. The future work needed to extend the Pitzer model to trace metals is discussed.
Similar content being viewed by others
References
Anderson, M. A. and Morel, F. M. (1982) The influence of aqueous iron chemistry on the uptake of iron by the coastal diatom Thalassiosira Weissflogii. Limnol. Oceanogr. 27, 789-813.
Atkinson, G., Dayhoff, M. O., and Ebdon, D. W. (1973) Computer modeling of inorganic equilibria in seawater. In: Marine Electrochemistry(eds. J. Berkowitz et al.), The Electrochemistry Soc., pp. 124-138.
Bates, R. G. (1951) J. Res. Nat. Bur. Std. 47, 127.
Bates, R. G and Acree, S. F. (1943) pH values of certain phosphate chloride mixtures and the second dissociation constant of phosphoric acid from 0 to 60 °C. J. Res Natl. Bur. Stand. 30, 129-155.
Bates, R. G. and Pinching, G. D. (1949) Acidic dissociation constant of ammonium ion at 0 to 50 °C, and the base strength of ammonium. J. Res. Natl. Bur. Stand. 42, 419-430.
Byrne, R. H. Jr. and Kester, D. R. (1974) Inorganic speciation of boron in seawater. J. Mar. Res. 32, 119-127.
Byrne, R. H., Kump, L. R., and Cantrell, K. J. (1988) The influence of temperature and pH on trace metal speciation in seawater. Mar. Chem. 25, 163-181.
Campbell, D. M., Millero, F. J., Roy, R., Roy, L., Lawson, M, Vogel, K. M., and Moore, C. P. (1993) The standard potential for the hydrogen - silver, silver chloride electrode in synthetic seawater. Mar. Chem. 44, 221-233.
Clegg, S. L. and Brimblecombe, P. (1988) Hydrofluoric and hydrochloric acid behavior in concentrated saline solutions. J. Chem. Soc. Dalton Trans. 705-710.
Clegg, S. L. and Brimblecombe, P. (1989) Solubility of ammonia in pure aqueous and multicomponent solutions. J. Phys. Chem. 93, 7237-7248.
Clegg, S. L. and Whitfield, M. (1991) Activity coefficients in natural waters. In: Activity Coefficients in Electrolyte Solutions(ed. K. S. Pitzer), CRS, Boca Raton, FL, pp. 279-434.
Clegg, S. L., Rard, J. A., and Pitzer K. S. (1994) Thermodynamic properties of 0-6 mol kg-1 aqueous sulphuric acid from 273.15 to 328.15 K. J. Chem. Soc. Faraday Trans. 90, 1875-1894.
Clegg, S. L. and Whitfield, M. (1995) A chemical model of seawater including dissolved ammonia and the stoichiometric dissociation constant of ammonia in estuarine water and seawater from -2 to 40 °C. Geochim. Cosmochim. Acta 59, 2403-2421.
Crerar, D. A. (1975) A method for computing multicomponent chemical equilibria based on equilibrium constants. Geochim. Cosmochim. Acta 39, 1375-1384.
Criss, C. and Millero, F. J. (1996) Modeling the heat capacities of aqueous 1-1 electrolyte solutions with Pitzer's equations. J. Phys. Chem. 91, 1288-1294.
Criss, C. and Millero, F. J. (1998) Modeling the heat capacities of aqueous 2-1 and 3-1 electrolyte solutions with Pitzer's equations. J. Solution Chem. submitted.
Culberson, C. and Pytkowicz, R. M. (1973) Ionization of water in seawater. Mar. Chem. 1, 309-316.
Culberson, C., Pytkowicz, R. M., and Hawley, J. E. (1970) Seawater alkalinity determination by the pH method. J. Mar. Res. 28, 15-21.
Culberson, C., Latham, G., and Bates, R. G. (1978) Solubilities and activity coefficients of calcium and strontium sulfates in synthetic seawater at 0.5 and 25 °C. J. Phys. Chem. 82, 2693-2699.
Davies, C. W. (1962) Ion Association, Butterworths, London, 190 pp.
Dickson, A. G. (1990a) Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep-Sea Res. 37, 755-766.
Dickson, A. G. (1990b) Standard potential of the reaction: AgCl(s) + 1/2 H2(g) = Ag(s) + HCl(aq), and the standard acidity constant of the HSO4 - in synthetic sea water from 273.15 to 318.15 K. J. Chem. Thermodyn. 22, 113-127.
Dickson, A. G. and Riley, J. P. (1979a). The estimation of acid dissociation constants in seawater from potentiometric titrations with strong base. I, The ion product of water - K W. Mar. Chem. 7, 89-99.
Dickson, A. G. and Riley, J. P. (1979b). The estimation of acid dissociation constants in seawater from potentiometric titrations with strong base. II, The dissociation of phosphoric acid. Mar. Chem. 7, 101-109.
Dickson, A. G. and Whitfield, M. (1981) An ion-association model for estimating acidity constants (at 25 °C and 1 atm total pressure) in electrolyte mixtures related to seawater (ionic strength <1 mol Kg-1 H2O). Mar. Chem. 10, 315-333.
Dickson, A. G. and Millero, F. J. (1987). A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 34, 1733-1743.
Dyrssen, D. and Wedborg, M. (1974) Equilibrium calculations of the speciation of elements in seawater. In: The Sea(ed. E. D. Goldberg), Wiley International, New York, pp. 181-195.
Dyrssen, D., Jagner, D., and Wengelin, F. (1968) Computer Calculations of Ionic Equilibria and Titration Procedures. Almqvist and Wiksell, Stockholm, 250 pp.
Elgquist, B. (1970) Determination of the stability constant of MgF+ and CaF+ using a fluoride ion selective electrode. J. Inorg. Nucl. Chem. 32, 937-944.
Felmy, A. R. and Weare, J. H. (1986) The prediction of borate mineral equilibria in natural waters: Application to Searles Lake, California. Geochim. Cosmochim. Acta 50, 2771-2783.
Garrels, R. M. and Thompson, M. E. (1962) A chemical model for seawater at 25 °C and one atmosphere total pressure. Amer. J. Sci. 260, 57-66.
Gieskes, J. M. T. (1966) The activity coefficients of sodium chloride in mixed electrolyte solutions at 25 °C. Physik. Chemie. Neue Folge 50, 78-90.
Goldberg, R. N. and Parker, V. B. (1985) Thermodynamics of solution of SO2(g) in water and of aqueous sulfur dioxide solutions. J. Res. National Bureau of Standards 90, 341-358.
Goyet, C. and Poisson, A. (1989) New determination of carbonic acid dissociation constants in seawater as a function of temperature and salinity. Deep-Sea Res. 36, 1635-1654.
Greenberg, J. P. and Møller, N. (1989) The prediction of mineral solubilities in natural waters: A chemical equilibrium model for the Na-K-Ca-Cl-SO4-H2O system to high concentration from 0 to 250 °C. Geochim. Cosmochim. Acta 53, 2503-2518.
Guggenheim, E. A. (1935) Thermodynamic properties of aqueous solutions of strong electrolytes. Phil. Mag. 19, 588-643.
Hansson, I. (1972) An Analytical Approach to the Carbonate System in Seawater. Ph.D. Thesis, University of Götenborg, Sweden.
Hansson, I. (1973). A new set of acidity constants for carbonic acid and boric acid in seawater. Deep-Sea Res. 20, 461-478.
Harned, H. S. and Scholes, S. R. (1941) The ionization constant of HCO-3 from 0 to 50 °C. J. Am. Chem. Soc. 63, 1706-1709.
Harned, H. S. and Davis, R. (1943) The ionization of carbonic acid in water and the solubility of carbon dioxide in water and aqueous salt solutions form 0 to 50 °C. J. Amer. Chem. Soc. 60, 2030-2037.
Harned, H. S. and Owen, B. B. (1958) The Physical Chemistry of Electrolytic Solutions, A.C.S. Monograph Reinhold Pub. Corp., London, p. 748.
Harvie, C. E. and Weare, J. H. (1980) The prediction of mineral solubilities in natural waters: the Na-K-Mg-Ca-SO4-Cl-H2O system from zero to high concentration at 25 °C. Geochim. Cosmochim. Acta 44, 981-997.
Harvie, C. E., Møller, N., and Weare, J. H. (1984) The prediction of mineral solubilities in natural waters: The Na-K-Mg-Ca-H-Cl-SO4-OH-HCO3-CO3-CO3-H2O system to high ionic strengths at 25 °C. Geochim. Cosmochim. Acta 48, 723-752.
He, S. and Morse, J. W. (1993) The carbonic acid system and calcite solubility in aqueous Na-K-Ca-Mg-Cl-SO4solutions from 0 to 90 °C. Geochim. Cosmochim. Acta 57, 3533-3554.
Hershey, P. J. and Millero, F. J. (1986) The dependence of the acidity constants of silicic acid on NaCl concentration using Pitzer's equations. Mar. Chem. 18, 101-105.
Hershey, P. J., Fernandez, M., Milne, P. J., and Millero, F. J. (1986) The ionization of boric acid in NaCl, Na-Ca-Cl and Na-Mg-Cl solutions at 25 °C. Geochim. Cosmochim. Acta 50, 137-148.
Hershey, P. J., Plese, T., and Millero, F. J. (1988) The pK1 for the ionization of H2S in various ionic media. Geochim. Cosmochim. Acta 52, 2047-2051.
Hershey, P. J., Millero, F. J., and Fernandez, M. (1989) The ionization of phosphoric acid in NaCl and NaMgCl solutions at 25 °C. J. Solution Chem. 18, 875-892.
Hovey, J. K., Pitzer, K. S., and Rard, J. A. (1993) Thermodynamics of aqueous sodium sulfate from 273 to 373 K and mixtures of aqueous sodium sulfate and sulfuric at 298 K. J. Chem. Thermodyn. 25, 173-192.
Ingri, N., Kakolowicz, W., Sillen, L. G., and Warnqvist, B. (1967) HALTAFALL, a general program for calculating the composition of equilibrium mixtures. Talanta 14, 1261-1286.
Johansson, O. and Wedborg, M. (1980) The ammonia-ammonium equilibrium in seawater at temperatures between 5 and 25 °C. J. Solution Chem. 9, 37-44.
Johnson, K. E. and Pytkowicz, R. M. (1981) The activity of NaCl in seawater of 10-40¡ salinity and 5-25 °C at 1 atmosphere. Mar. Chem. 10, 85-91.
Kester, D. R. and Pytkowicz, R. M. (1967) Determination of the apparent dissociation constants of phosphoric acid in seawater. Limnol. Oceanogr. 12, 243-252.
Kielland, J. (1937) Individual activity coefficients of ions in aqueous solutions at 25 °C and 1 atmosphere. J. Am. Chem. Soc. 59, 1675-1678.
Knowles, G. and Wakeford, A. C. (1978) A mathematical deterministic river-quality model. Part 1: Formulation and description. Water Res. 12, 1149-1153.
Khoo, K. H., Ramette, R.W., Culberson, C. H., and Bates, R. G. (1977a) Determination of hydrogen ion concentrations in seawater from 5 to 40 °C: standard potentials at salinities from 20 to 45¡. Anal. Chem. 49, 29-34.
Khoo, K. H., Culberson, C. H., and Bates, R. G. (1977b) Thermodynamics of ammonium ion in seawater from 5 to 40 °. J. Solution Chem. 6, 281-290.
Leyendekkers, J. V. (1972) The chemical potential of seawater components. Mar. Chem. 1, 75-88.
Manov, G. G., DeLollis, N. J., and Acree, S. F. (1944) Ionization constant of boric acid and the pH of certain borax-chloride buffer solutions from 0 to 60 °C. J. Res. Nat. Bur. Stds. 33, 287-306.
Mehrbach, C., Culberson, C. H., Hawley, J. E., and Pytkowicz, R. M. (1973). Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897-907.
Millero, F. J. (1975) The physical chemistry of estuaries, Chapter 2. In: Marine Chemistry in the Coastal Environment(ed. T. M. Church), ACS Symp. Ser. 18, Washington, D.C., pp. 25-55.
Millero, F. J. (1979a) Effect of temperature and pressure on activity coefficients. In: Activity Coefficients in Electrolyte Solutions(ed. R. M. Pytkowicz), Vol. II. CRC Press, Boca Raton, Fl., (1979), pp. 63-151.
Millero, F. J. (1979b) The thermodynamics of the carbonate system in seawater. Geochim. Cosmochim. Acta 43, 1651-1661.
Millero, F. J. (1981) The ionization of acids in estuarine waters. Geochim. Cosmochim. Acta 45, 2085-2089.
Millero, F. J. (1982) Use of models to determine ionic interactions in the natural waters. Thalassia Jugoslavica 1-4, 253-291.
Millero, F. J. (1983). The estimation of the pK° HA of acids in seawater using the Pitzer equations. Geochim. Cosmochim. Acta. 47, 2121-2129.
Millero, F. J. (1984) The activity of metal ions at high ionic strengths. In: Complexation of Trace Metals in Natural Waters(eds. C. J. M. Kramer and J. C. Duinker), Martinus Nijhoff/W. Junk, The Hague, pp. 187-200.
Millero, F. J. (1985) The physical chemistry of natural waters. J. Pure and Appl. Chem. 57, 1015-1024.
Millero, F. J. (1986) The thermodynamics and kinetics of the hydrogen sulfide system in natural waters. Mar. Chem. 18, 121-147.
Millero, F. J. (1990a) Effect of speciation on the rates of oxidation of metals. In: Chemical Modeling in Aqueous Systems II(eds. D. Melchior and R. Bassett), ACS Books, Chapter 34, Washington D.C., pp. 447-460.
Millero, F. J. (1990b) Marine solution chemistry and ionic interactions. Mar. Chem. 30, 205-229.
Millero, F. J. (1992) Stability constants for the formation of rare earth inorganic complexes as a function of ionic strength. Geochim. Cosmochim. Acta 56, 3123-3132.
Millero, F. J. (1995) Thermodynamics of carbon dioxide system in the oceans. Geochim. Cosmochim. Acta 59, 661-677.
Millero, F. J. and Leung, W. H. (1976) The thermodynamics of seawater at one atmosphere. Amer. J. Sci. 276, 1035–1077.
Millero, F. J. and Schreiber, D. R. (1982) Use of the ion pairing model to estimate activity coefficients of the ionic components of natural waters. Amer. J. Sci. 282, 1508-1540.
Millero, F. J. and Thurmond, V. (1983) The ionization of carbonic acid in Na-Mg-Cl solutions at 25 °C. J. Solution Chem. 12, 401–412.
Millero, F. J. and Byrne, R. H. (1984) Use of Pitzer's equations to determine the media effect on the formation of lead chloro complexes. Geochim. Cosmochim. Acta 48, 1145-1150.
Millero, F. J. and Hawke, D. J. (1992) Ionic interactions of divalent metals in natural waters. Mar. Chem. 40, 19-48.
Millero, F. J., and Roy, R. (1997) A chemical model for the carbonate system in natural waters. Croatia Chemica Acta 70, 1-38.
Millero, F. J., Hershey, J. P., and Fernandez, M. (1987) The pK★ of TRISH+ in Na-K-Mg-Ca-Cl-SO4brines-pH scales. Geochim. Cosmochim. Acta 51, 707-711.
Millero, F. J., Plese, T., and Fernandez, M. (1988) The dissociation of hydrogen sulfide in seawater. Limnol. Oceanogr. 33, 269-274.
Millero, F. J., Hershey, J. P., Johnson, G., and Zhang, J. (1989) The solubility of SO2 and the dissociation of H2SO3 in NaCl solutions. J. Atmos. Chem. 8, 377-389.
Millero, F. J., Yao, W., and Aicher, J. (1995) The speciation of Fe(II) and Fe(III) in natural waters. Mar. Chem. 50, 21-39.
Møller, N. (1988) The prediction of mineral solubilities in natural waters: A chemical equilibrium model for the Na-Ca-Cl-SO4-H2O system, to high temperature and concentration. Geochim. Cosmochim. Acta 52, 821-837.
Morel, F. and Morgan, J. (1972) A numerical method for computing equilibria in aqueous chemical systems. Environ. Sci. Technol. 658-67.
Mucci, A. (1983) The solubility of calcite and aragonite in seawater at various salinities, temperatures and one atmosphere total pressure. Amer. J. Sci. 283, 780-799.
Nordstrom, D. K. and Ball, J.W. (1984) Chemical models, computer programs and metal complexation in natural water. In: Complexation of Trace Metals in Natural Waters(eds. C. J. M. Kramer and J. C. Duinker), Martinus Nijhoff/W. Junk, The Hague, pp. 149-162.
Novak, S. F., Al Mahamid, I., Becraft, K. A., Carpenter, S.A., Hakem, N., and Prussin, T. (1997) Measurement and thermodynamic modeling of Np(V) solubility in aqueous K2CO3 solutions to high concentrations. J. Solution Chem. 26, 681-697.
Owen, B. B. (1934) The dissociation constant of boric acid from 10 to 50°C. J. Am. Chem. Soc. 56, 1695-1697.
Owen, B. B. and King, E. J. (1943) The effect of sodium chloride upon the ionization of boric acid at various temperatures. J. Amer. Chem. Soc. 65, 1612-1620.
Pabalan, R. T. and Pitzer, K. (1987) Thermodynamics of concentrated electrolyte mixtures and the prediction of mineral solubilities to high temperature for mixtures in the system Na-K-Mg-Cl-SO4-OH-H2O. Geochim. Cosmochim. Acta 51, 2429-2443.
Peiper, J. C. and Pitzer, K. S. (1982) Thermodynamics of aqueous carbonate solutions including mixtures of sodium carbonate, bicarbonate and chloride. J. Chem. Thermodyn. 14, 613-638.
Perrin, D. D. and Sayce, I. G. (1967) Computer calculation of equilibrium concentration in mixtures of metal ions and complexing species. Talanta 12, 833-842.
Pierrot, D., Millero, F. J., Roy, L. N., Roy, R. N., Doneski, A., and Niederschmidt, J. (1997) The activity coefficients of HCl in HCl-Na2SO4 solutions from 0 to 50 °C and I= 6 m. J. Solution Chem. 26, 31-45.
Pierrot, D., Millero, F. J., Roy, L. N., Roy, R. N., Doneski, A., and Smithson, J. (1998) The activity coefficients of HCl in HCl-K2SO4, and HCl-MgSO4 solutions from 0 to 50 °C and I= 6 m. J. Solution Chem.submitted.
Pitzer, K. S. (1973) Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys. Chem. 77, 268-277.
Pitzer, K. S. (1975) Thermodynamics of electrolytes. V. Effects of higher order electrostatic terms. J. Solution Chem. 3, 249-265.
Pitzer, K. S. (1979) Theory: ion interaction approach. In: Activity Coefficients in Electrolyte Solutions(ed. R. M. Pytkowicz), Vol. I. CRC Press, Boca Raton, Fl., pp. 157-208.
Pitzer, K. S. (1991) Theory: ion interaction approach: theory and data collection. In: Activity Coefficients in Electrolyte Solutions(ed. K. S. Pitzer), 2nd Ed., Vol. I, CRC Press, Boca Raton, Fl., pp. 75-153.
Pitzer, K. S. and Mayorga, G. (1973) Thermodynamics of electrolytes. II. activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J. Phys. Chem. 77, 2300-2308.
Pitzer, K. S. and Mayorga, G. (1974) Thermodynamics of electrolytes. III. Activity and osmotic coefficients for 2-2 electrolytes. J. Solution Chem. 3, 539-546.
Pitzer, K. S. and Kim, J. J. (1974) Thermodynamics of electrolytes. IV. Activity and osmotic coefficients for mixed electrolytes. J. Am. Chem. Soc. 96, 5701-5707.
Platford, R. F. (1965) The activity coefficient of sodium chloride in seawater. J. Mar. Res. 23, 55-62.
Platford, R. F. and Dafoe, T. (1965) The activity coefficient of sodium sulfate in seawater. J. Mar. Res. 23, 63-68.
Roy, R. N., Gibbons, J. J., Bliss, D. P., Jr., Casebolt, R. G., and Baker, B. K. (1980) Activity coefficients for ternary systems: VI. The system HCl + MgCl2 + H2O at different temperatures; application of Pitzer's equations. J. Solution Chem. 9, 911-929.
Roy, R. N., Gibbons, J. J., Ovens, L. K., Bliss, G. A., and Hartley, J. J. (1982a) VII. Activity coefficients for the system HCl + CaCl2 + H2O at various temperatures. J. Chem. Soc. Faraday Trans. 78, 1405-1422.
Roy, R. N., Gibbons, J. J., Trower, J. K., Lee, G. A., Hartley, J. J., and Mack, J. G. (1982b) The ionization of carbonic acid in solutions of sodium chloride from e.m.f measurements at 278.15, 298.15, and 318.15 K. J. Chem. Thermodyn. 14, 473-482.
Roy, R. N., Gibbons, J. J., Wood, M. D., Williams, R. W., Peiper, J. C., and Pitzer, K. S. (1983) The first ionization of carbonic acid in aqueous solutions of potassium chloride including the activity coefficients of potassium bicarbonate. J. Chem. Thermodyn. 15, 37-47.
Roy, R. N., Gibbons, J. J., Roy, L. N., and Greene, M. A. (1986) Thermodynamics of the unsymmetrical mixed electrolyte HCl-SrCl2. Applications of Pitzer's equations. J. Phys. Chem. 90, 6242-6247.
Roy, R. N., Zhang, J. Z., and Millero, F. J. (1991) The ionization of sulfurous acid in Na-Mg-Cl Solutions at 25 °C. J. Solution Chem. 20, 361-373.
Roy, R. N., Roy, L. N., Lawson, M., Vogel, K. M., Porter-Moore, C., Davis, W., Millero, F. J., and Campbell, D. M. (1993) The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45 °C. Mar. Chem. 44, 249-259.
Rush, R. M. and Johnson, J. S. (1966). Osmotic coefficients of synthetic seawater solutions at 25 °C. J. Chem. Eng. Data 11, 590-592.
Sillen, L. G. (1961) The physical chemistry of seawater. In: The Sea(ed. S. Sears), Oceanography. Amer. Assoc. Adv. Sci., Publication 67, pp. 549-581. Washington D.C.
Sillen, L. G. and Martell, A. E. (1964) Stability Constants of Metal-ion Complexes, The Chemical Society, London, 754 pp. Silvester, L. F. and Pitzer, K. S. (1978) Thermodynamic of electrolytes. X. Enthalpy and the effect of temperature on the activity coefficients. J. Solution Chem. 7, 327-337.
Simonson, J. M., Roy, R. N., and Gibbons, J. J. (1987a) Thermodynamics of aqueous mixed potassium carbonate, bicarbonate, and chloride solutions to 368 K. J. Chem. Eng. Data 32, 41-45.
Simonson, J. M., Roy, R. N., Connole, J., Roy, L. N., and Johnson, D. A. (1987b) The thermodynamics of aqueous borate solutions. II. Mixtures of boric acid with calcium or magnesium borate and chloride. J. Solution Chem. 16, 791-803.
Simonson, J. M., Roy, R N., Mrad, D., Lord, P., Roy, L. N., and Johnson, D. A. (1988) The thermodynamics of aqueous borate solutions, I. Mixtures of boric acid with sodium or potassium borate and chloride. J. Solution Chem. 17, 435-446.
Spencer, R. J., Møller, N., and Weare, J. H. (1990) The prediction of mineral solubilities in natural waters; a chemical equilibrium model for the Na-K-Ca-Mg-Cl-SO4-H2O system at temperatures below 25 °C. Geochim Cosmochim Acta 54, 575-590.
Thompson, M. E. (1966) Magnesium in sea water: an electrode measurement, Science 153, 866-867.
Thurmond, V. L. and Millero, F. J. (1982) Ionization of carbonic acid in sodium chloride solutions. J. Solution Chem. 11, 447-456.
Truesdell, A. H. and Jones, B. F. (1969) Ion association of natural brines. Chem. Geol. 4, 51-62.
Turner, D. R., Whitfield, M., and Dickson, A. G. (1981) The equilibrium speciation of dissolved components in freshwater and seawater at 25 °C and 1 atm pressure. Geochim. Cosmochim. Acta 45, 855-881.
Turner, D. R. and Whitfield, M. (1987) An equilibrium speciation model for copper in sea and estuarine waters at 25 °C including complexation with glycine, EDTa and NTA. Geochim. Cosmochim. Acta 51, 3231-3239.
van Breeman, N. (1973) Calculation of activity coefficients in natural waters. Geochim. Cosmochim. Acta 37, 101-107.
Weiss, R. (1974). Carbon dioxide in water and seawater. The solubility of a non-ideal gas. Mar. Chem. 2, 203-215.
Westall, J. C., Zachary, J. L., and Morel, F. M. M. (1976) MINEQL: A computer program for the calculation of chemical equilibrium composition of aqueous systems. Tech. Note No. 18, School of Engineering, Massachusetts Institute of Technology.
Whitfield, M. (1973) A chemical model for the major electrolyte component of seawater based on the Brønsted-Guggenheim hypothesis. Mar. Chem. 1, 251-266.
Whitfield, M. (1975a) An improved specific interaction model for seawater at 25 °C and one atmosphere total pressure. Mar. Chem. 3, 197-213.
Whitfield, M. (1975b) The extension of chemical models for seawater to include trace components. Geochim. Cosmochim. Acta 39, 1545-1557.
Whitfield, M. (1979) Activity coefficients in natural waters. In: Activity Coefficients in Electrolyte Solutions(ed. R. M. Pytkowicz), Vol. II. CRC Press, Boca Raton, Fl., pp. 153-299.
Yao, W. and Millero, F. J. (1995) The chemistry of the anoxic waters in the Framvaren Fjord, Norway. Aquatic Chem. 1, 53-88.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Millero, F.J., Pierrot, D. A Chemical Equilibrium Model for Natural Waters. Aquatic Geochemistry 4, 153–199 (1998). https://doi.org/10.1023/A:1009656023546
Issue Date:
DOI: https://doi.org/10.1023/A:1009656023546