Abstract
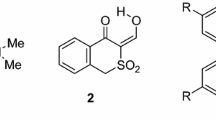
The reaction of 5-oxotetrahydro-4H-thiochromenes with Grignard reagents has been studied and it has been shown that the reagent adds to the 1,2-position of the conjugated system in the cyclic ketones, The structure of the products depends on that of the starting cyclic ketone.
Similar content being viewed by others
References
N. S. Smirnova, L. I. Lelyukh, K. M. Korshunova, I. Ya. Evtushenko, and V. G. Kharchenko, Zh. Org. Khim.,10, 194 (1974).
H. D. House, W. L. Respess, and L. M. Whitesides, J. Org. Chem.,31, 3128 (1966).
D. Yamamoto, M. Yanagisawa, U. Tomita, T. Ogawa, L. Senga, K. Hagamizi, and F. Taka, J. Nat. Chem. Lab.,68, 95 (1973).
V. G. Kharchenko, L. I. Markova, and K. M. Korshunova, Zh. Org. Khim., No. 12, 663 (1976).
Additional information
N. G. Chernyshevskii State University, Saratov 410026. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 5, pp. 613–615, May, 1994. Original article submitted April 22, 1994.
Rights and permissions
About this article
Cite this article
Markova, L.I., Kharchenko, V.G. Specific reactions of 5-oxotetrahydro-4H-thiochromenes with Grignard reagents. Chem Heterocycl Compd 30, 537–539 (1994). https://doi.org/10.1007/BF01169828
Issue Date:
DOI: https://doi.org/10.1007/BF01169828