Abstract
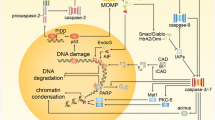
Apoptosis induced by etoposide (VP-16) in HL-60 cells was confirmed to be caspase-dependent. It was fully inhibited by the broad-spectrum caspase inhibitor Z-VAD-fmk. However, the caspase-3-specific inhibitor Z-DEVD-fmk only partially inhibited apoptosis. This indicated that a second caspase is required in vivo for full activation of the apoptotic nucease CAD. Aurin tricarboxylic acid (ATA) did not inhibit VP-16-induced apoptosis. In contrast, apoptosis induced by hydroxychloroquine (HCQ) in HL-60 cells was caspase-3 independent and was fully inhibited by ATA. Thus, CAD does not appear to be involved in chromatin DNA degradation in this case. A second apoptotic nuclease is postulated to degrade the DNA, likely endo-exonuclease, an abundant nuclear enzyme that acts on both DNA and RNA and is present in latent form. HCQ, but not VP-16, stimulated DNA degradation (“laddering”) in isolated nuclei. This indicates that the drug can act directly in the nuclei to trigger activation of the second latent apoptotic nuclease.
Similar content being viewed by others
References
Cohen GM. Caspases: the executioners of apoptosis. Biochem J 1997; 326: 1–16.
Enari M, Sakahira H, Yokoyama K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis. Nature 1998; 391: 43–50.
Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 1998: 391; 96–99.
Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to triggerDNA fragmentation during apoptosis. Cell 1997; 89: 175–184.
Liu X, Li P, Widlak P, Zou H, Luo X, Garrard WT, Wang X. The 40–kDA subunit of DNA fragmentation factor induces DNAfragmentation and chromatin condensation during apoptosis, Proc Natl Acad Sci USA 1998; 95: 8461–8466.
Mukae N, Enari M, Sakahira H, et al. Molecular cloning and characterization of human caspase-activated DNase. Proc Natl Acad Sci USA 1998; 95; 9123–9128.
Zamzami N, Susin S, Marchetti P, et al. Mitochondrial control of nuclear apoptosis. J Exp Med 1996; 183: 1533–1544.
Susin S, Zamzami N, Castedo M, et al. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95–and ceramide-induced apoptosis. J Exp Med 1997; 186: 25–37.
Susin S, Lorenzo HK, Zamzami N, et al. Mitochondrial release of caspase-2 and-9 during the apoptotic process. J Exp Med 1999; 189: 381–394.
Krippner A, Matsuno-Yagi A, Gottlieb RA, Babior BM. Loss of function of cytochrome c in Jurkat cells undergoing Fasmediated apoptosis. J Biol Chem 1996; 271: 21629–21636.
Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 1996; 89: 175–184.
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 1997; 275: 1132–11136.
Kluck RM, Martin SJ, Hoffman BM, Zhou JS, Green DR, Newmeyer DD. Cytochrome c activation of CPP-32–like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO J 1997; 16: 4639–4649.
Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 1997; 275: 1126–1132.
Marzo I, Brenner C, Zamzami N, Susin S, et al. The permeability transition pore complex: a target for apoptosis regulation by caspases and Bcl-2–related proteins. J Exp Med 1998; 187: 1261–1271.
Zanzami N, Marchetti P, Castedo M, et al. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med 1995; 181: 1661–1672.
Meng XW, Fraser MJ, Ireland CM, Feller JM, Ziegler JB. An investigation of a possible role for mitochondrial nuclease in apoptosis. Apoptosis 1998; 3: 395–406.
Scaffidi C, Fuida S, Srinivasan A, et al. Two CD95 (APO-a/Fas) signaling pathways. EMBO J 1998; 17: 1675–1687.
Adjei PN, Kaufmann SH, Leung, et al. Selective induction of apoptosis in Hep 3B cells by topoisomerase I inhibitors: evidence for a protease-dependent pathwway that does not activate cysteine protease P32. J Clinic Invest 1996; 98: 2588–2596.
Lavoie JN, Nguyen M, Marcellus RC, Branton PE, Shore GC. E4orf4, a novel adenovirus death factor that induces p53–independent apoptosis by a pathway that is not inhibited by Z-VAD-fmk. J Cell Biol 1998; 140: 637–645.
Monney L, Otter I, Olivier R, et al. Defects in the ubiquitin pathway induce caspase-independent apoptosis blocked by Bcl-2. J Biol Chem 1998; 273: 6121–6131.
Saeki K, Akira Y, Kato M, Miyazono K, Yakazaki Y, Takaku F. Cell density-dependent apoptosis in HL-60 cells, which is mediated by an unknown factor, is inhibited by transforming growth factor beta 1 and overexpression of Bcl-2. J Biol Chem 1997; 272: 20003–20010.
Martins LM, Kottke TJ, Messner PW, et al. Activation of multiple interleukin-1beta converting homologues in cytosol and nuclei of HL-60 cells during etoposide-induced apoptosis. J Biol Chem 1997; 272: 7421–7430.
McConkey DJ, Hartzell P, Nicotera P, et al. Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J 1989; 3: 1843–1849.
Fraser MJ, Low RL. Fungal and mitochondrial nucleases. In: Roberts RJ, Linn SM, Lloyd RS, eds. Nucleases, Second Edition. Cold Spring Harbor Laboratory Press 1993: 171–207.
Fraser MJ, Tynan SJ, Papaioannou A, Ireland CM, Pittman SM. Endo-exonuclease of human leukaemic cells: evidence for a role in apoptosis. J Cell Sci 1996; 109: 2343–2360.
Martins LM, Messner PW, Kottke TJ, et al. Comparison of caspase activation and subcellular localization in HL-60 and K562 cells undergoing etoposide-induced apoptosis. Blood 1998; 90: 4283–4296.
Srinivasula SM, Fernandes-Alnemri T, Zangrilli J, et al. The CED-3/interleukin-1¯-converting enzyme-like homolog Mch6 and the lamin-cleaving enzyme Mch2 are substrates for the apoptotic mediator CPP32. J Biol Chem 1996; 271: 27099–27106.
Zvaifler NJ. The subcellular localization of chloroquine and its effect on lysosomal disruption. Arthritis Rheum 1964; 7: 760–761.
Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol 1981; 90: 665–669.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meng, X.W., Fraser, M.J., Feller, J.M. et al. Caspase-3-dependent and caspase-3-independent pathways leading to chromatin DNA fragmentation in HL-60 cells. Apoptosis 5, 61–67 (2000). https://doi.org/10.1023/A:1009689710184
Issue Date:
DOI: https://doi.org/10.1023/A:1009689710184