Abstract
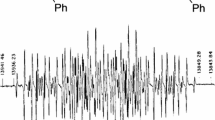
195Pt NMR on the organic ligand stabilized metal cluster compound Pt309Phen *36 O30 reveals two separate peaks in the lineshape. Ligand-bonded platinum atoms at the surface of the core are thought to be responsible for the peak that does not show any Knight shift. The corresponding spin-lattice relaxation timeT 1 is of the order of seconds. The second peak is Knight shifted and is attributed to the other Pt atoms, for which metallic behavior is inferred from the temperature dependence ofT 1. The Korringa relation holds down to 65 K. Below 65 K the relaxation of the magnetization becomes increasingly non-exponential with decreasing temperature. The relaxation process can be successfully modelled under the assumption of a Poisson distribution of the energy levels around the Fermi energy (the electronic quantum size effect).
Similar content being viewed by others
References
Slichter C.P.: Surf. Sc.106, 373 (1981).
Rhodes, H.E., Wang, P.K., Stokes, H.T., Schlichter, C.P., Sinfelt, J.H.: Phys. Rev.B26, 3359 (1982).
Rhodes, H.E., Wang, P.K., Makowka, C.D., Rudaz, S.L., Stokes, H.T., Slichter, C.P., Sinfelt, J.H.: Phys. Rev.B26, 3569 (1982).
Stokes, H.T., Rhodes, H.E., Wang, P-K., Slichter, C.P., Sinfelt, J.H.: Phys. Rev.B 26, 3575 (1982).
Makowka, C.D., Slichter, C.P., Sinfelt, J.H.: Phys. Rev.B31, 5663 (1985).
Ansermet, J-P., Wang, P-K, Slichter, C.P., Sinfelt, J.H.: Phys. Rev.B 37, 1417 (1988).
Yu, I., Halperin, W.P.: J. Low. Temp. Phys.45, 189 (1981)
Van der Klink, J.J., Buttet, J., Graetzel, M.: Phys. Rev.B 29, 6352 (1984).
Bucher, J.P., Van der Klink, J.J.: Phys. Rev.B38, 11038 (1988).
Bucher, J.P., Buttet, J., Van der Klink, J.J., Graetzel, M.: Surface Science214, 347 (1989)
Van der Klink, J.J.: Physics and Chemistry of Finite Systems: From Clusters to Crystals, volI, 537 (1992), P. Jena et al. (eds.), Kluwer Academic Publ.
Pronk, B.J., Brom, H.B., Ceriotti, A., Longoni, G.: Solid State Commun. bf 64, 7 (1987).
Van Staveren, M.P.J., Brom, H.B., de Jongh, L.J., Schmid, G.: Z. Phys. D12, 451 (1989); id.: Z. Phys. D.20, 333 (1991)
Van der Putten, D., Brom, H.B., de Jongh, L.J., Schmid, G.: Physics and Chemistry of finite systems: From Clusters to Crystals, volII, 1007 (1992), P. Jena et al. (eds.), Kluwer Academic Publ.
Goto, T., Komori, F., Kobayashi, S.: J. Phys. Soc. Jap.58, 3788 (1989).
Kobayashi, S., Goto, H., Katsumoto, S.: J. Phys. Soc. Jap. 61, 1856 (1992)
Kubo, R.: J. Phys. Soc. Japan17, 976 (1962)
Halperin, W.P.: Rev. Mod. Phys.58, 553 (1986)
Perenboom, J.A.A.J., Wyder, P., Meier, F.: Phys. Rep.78, 173 (1981)
Denton, R., Mühlschlegel, B., Scalapino, D.J.: Phys. Rev.B 7, 3589 (1973).
Gor'kov, L.P., Eliashberg, G.M.: Sov. Phys. JETP21, 940 (1965)
Baak, J., Brom, H.B., de Jongh, L.J., Scmid, G.: (this conference) to be published in Z. Phys. D.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van der Putten, D., Brom, H.B., Witteveen, J. et al. The electronic quantum size effect observed by195Pt NMR in the metal cluster compound Pt309Phen *36 O30 . Z Phys D - Atoms, Molecules and Clusters 26 (Suppl 1), 21–23 (1993). https://doi.org/10.1007/BF01425605
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01425605