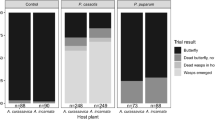
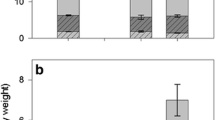
Summary
A number of aposematic butterfly and moth species sequester toxic substances from their host plants. Some of these insects can detect the toxic compounds during food assessment. Some pipevine swallowtails use aristolochic acids among the host finding cues during oviposition and larval feeding and accumulate the toxins in the body tissues throughout all life stages. Likewise, a danaine butterfly,Idea leuconoe, which sequesters high concentrations of pyrrolizidine alkaloids in the body, lays eggs in response to the specific alkaloid components contained in the apocynad host. Insect species sharing the same poisonous host plants may differ in the degree of sequestration of toxins. Two closely ralated aposematic geometrid moth species,Arichanna gaschkevitchii andA. melanaria, sequester a series of highly toxic diterpenoids (grayanotoxins) in different degrees, while a cryptic geometrid species,Biston robstus, does not sequester the toxins, illustrating the diversity in adaptation mechanisms even within the same subfamily. By contrast, a number of lepidopteran species store the same compounds though feeding upon taxonomically diverse plant species. A bitter cyanoglycoside, sarmentosin, was characterised from several moth species in the Geometridae, Zygaenidae and Yponomeutidae, and from the apollo butterflies,Parnassius spp. (Papilionidae), although each species feeds on different groups of plants.
Interspecific similarities and differences in life history and ecology are discussed in relation to variable characteristics of sequestration of plant compounds among these lepidopteran insects.
Similar content being viewed by others
References
Abe F, Yamauchi T (1987) Parsonine, a pyrrolizidine alkaloid fromParsonsia laevigata. Chem Pharm Bull 35:4661–4663
Abe F, Nagao T, Okabe H, Yamauchi T, Marubayashi N, Ueda I (1990) Parsonsianine, a macrocyclic pyrrolizidine alkaloid from the leaves ofParsonsia laevigata (Studies onParsonsia. III). Chem Pharm Bull 38:2127–2129
Abe F, Yamauchi T, Yaga S, Minato K (1991) Pyrrolizidine alkaloids fromParsonsia laevigata in Okinawa Island (Studies onParsonsia. V). Chem Pharm Bull 39:1576–1577
Aplin RT, Benn MH, Rothschild M (1968) Poisonous alkaloids in the body tissues of the cinnabar moth (Callimorpha jacobeae L.). Nature 219:747–748
Aplin RT, d'Archy Ward R, Rothschild M (1975) Examination of the large white and small white butterflies (Pieris spp.) for the presence of mustard oil and mustard oil glycosides. J Entomol 30:73–78
Blum MS (1981) Chemical Defenses of Arthropods. New York: Academic Press
Boppré M (1978) Chemical communication, plant relationships, and mimicry in the evolution of danaid butterflies. Entomol exp appl 24:264–277
Boppré M (1986) Insects pharmacophagously utilizing defensive plant chemicals (pyrrolizidine alkaloids). Naturwissenschaften 73:17–26
Boppré M (1990) Lepidoptera and pyrrolizidine alkaloids. Exemplification of complexity in chemical ecology. J Chem Ecol 16:165–185
Boros CA, Stermitz FR, McFarland N (1991) Processing of iridoid glycoside antirrinoside fromMaurandya antirrhiniflora (Scrophulariaceae) byMeris paradoxa (Geometridae) andLepipolys species (Noctuidae). J Chem Ecol 17:1123–1133
Bowers MD (1984) Iridoid glycosides and host-plant specificity in larvae of the buckeye butterfly,Junonia coenia (Nymphalidae). J Chem Ecol 11:1567–1577
Bowers MD, Collinge SK (1992) Fate of iridoid glycosides in different life stages of the buckeye,Junonia coenia (Lepidoptera: Nymphalidae). J Chem Ecol 18:817–831
Brower JVZ (1958) Experimental studies of mimicry in some North American butterflies. II.Battus philenor andPapilio troilus, P. polyxenes andP. glaucus. Evolution 12:123–136
Brower LP (1969) Ecological chemistry. Sci Am 220:22–29
Brower LP (1984) Chemical defence in butterflies. Pp 109–134in Vane-Wright RI, Ackery PR (eds) The Biology of Butterflies. Symp R Entomol Soc Lond 11. GB-London: Academic Press
Brown KS (1984) Adult-obtained pyrrolizidine alkaloids defend ithomiine butterflies against a spider predator. Nature 309:707–709
Conner WE, Eisner T, Van der Meer RK, Guerrero A, Meinwald J (1981) Precopulatory sexual interaction in an arctiid moth (Utetheisa ornatrix): role of a pheromone derived from dietary alkaloids. Behav Ecol Sociobiol 9:227–235
David WAL, Gardiner BOC (1962) Oviposition and the hatching of the eggs ofPieris brassicae (L.) in a laboratory culture. Bull Entomol Res 53:91–109
Davis RH, Nahrstedt A (1979) Linamarin and lotaustralin as the source of cyanide inZygaena filipendulae L. (Lepidoptera). Comp Biochem Physiol 69B:903–904
Davis RH, Nahrstedt A (1984) Cyanogenesis in insects. Pp 635–654in Kerkut GA, Gilbert LI (eds) Comprehensive Insect Physiology, Biochemistry and Pharmacology. II. Pharmacology. GB-Oxford: Pergamon Press
Dethier VG (1941) Chemical factors determining the choice of food plants byPapilio larvae. Am Nat 75:61–73
Dixon CA, Erickson JM, Kellett DN, Rothschild M (1978) Some adaptations betweenDanaus plexippus and its food plant, with notes onDanaus chrysippus andEuploea core (Insecta: Lepidoptera). J Zool (Lond) 185:437–467
Duffey SS (1980) Sequestration of plant natural products by insects. Annu Rev Entomol 25:447–477
Dussourd DE, Ubik K, Harvis C, Resch J, Meinwald J, Eisner T (1988) Biparental defensive endowment of eggs with acquired plant alkaloid in the mothUtetheisa ornatrix. Proc Natl Acad Sci 85:5992–5996
Dussourd DE, Harvis CA, Meinwald J, Eisner T (1989) Paternal allocation of sequestered plant pyrrolizidine alkaloids to eggs in the danaine butterfly,Danaus gilippus. Experientia 45:896–898
Edgar JA (1982) Pyrrolizidine alkaloids sequestered by Solomon Island danaine butterflies. The feeding preferences of the Danainae and Ithomiinae. J Zool (Lond) 196:385–399
Edgar JA (1984) Parsonsieae: ancestral larval foodplants of the Danainae and Ithomiinae. Pp 91–93in Vane-Wright RI, Ackery PR (eds) The Biology of Butterflies. Symp R Entomol Soc Lond 11. GB-London: Academic Press
Edgar JA, Culvenor CCJ, Robinson GS (1973) Hairpencil dihydropyrrolizines of Danainae from the New Hebrides. J Austr Entomol Soc 12:144–150
Edgar JA, Culvenor CCJ, Pliske TE (1974) Coevolution of danaid butterflies with their host plants. Nature 250:646–648
Edgar JA, Eggers NJ, Jones AJ, Russell GB (1980) Unusual macrocyclic pyrrolizidine alkaloids fromParsonsia heterophylla A. Cunn andParsonsia spiralis Wall. (Apocynaceae). Tetrahedron Lett 21:2657–2660
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Eisner T, Kluge AF, Ikeda MI, Meinwald YC, Meinwald J (1971) Defensive mechanisms of arthropods XXXIX. Sesquiterpenes in the osmeterial secretion of a papilionid butterffy,Battus polydamas. J Insect Physiol 17:245–250
EI-Naggar SF, Doskotch RW, Odell TM, Girard L (1980) Antifeedant diterpenes for the gypsy moth larvae fromKalmia latifolia: Isolation and characterization of ten grayanoids. J Nat Products 43:617–631
Euw von J, Reichstein T, Rothschild M (1968) Aristolochic acid-I in the swallowtail butterflyPachliopta aristolochiae Fabr. (Papilionidae). Isr J Chem 6:659–607
Fang SD, Yan XQ, Li CF, Fan ZY, Xu XR, Xu JS (1982) Studies on the chemical constituents ofSedum sarmentosum Bunge. IV. The structure of sarmentosin and isosarmentosin. Acta Chimica Sinica 40:273–280
Feeny P (1992) The evolution of chemical ecology: contribution from the study of herbivorous insects. Pp 1–44in Rosenthal GA, Janzen DH (eds) Herbivores: Their Interactions with Secondary Plant Metabolites. Vol I: The Chemical Participants. 2nd ed. New York: Academic Press
Feeny P, Rosenberry L, Carter M (1983) Chemical aspects of oviposition behavior in butterflies. Pp 27–76in Ahmad S (ed) Herbivorous Insects. New York: Academic Press
Frazer JFD, Rothschild M (1960) Defence mechanisms in warningly-coloured moth and other insects. Int Congr Entomol (11) 3:249–256
Guilford T, Nicol C, Rothschild M, Moore B (1987) The biological roles of pyrazines: evidence for a warning odour function. Biol J Linn Soc 31:113–128
Hikino H, Ogura M, Fushiya S, Konno C, Takemoto T (1977) Stereostructure of asebotoxin VI, VIII and IX, toxins ofPieris japonica. Chem Pharm Bull 25:523–524
Hirashima Y, Yano K, Chujo M (1974) Insect pest ofRhododendron kiusianum Makino (Ericaceae), with special reference to out-breaks ofInurois sp. andArichanna melanaria Linnaeus (Lepidotera, Geometridae) on Mts. Kuju and Kirishima. Sci Bull Fac Agric Kyushu Univ 29:87–115
Honda K (1980a) Odor of a papilionid butterfly. Odoriferous substances emitted byAtrophaneura alcinous alcinous (Lepidoptera: Papilionidae). J Chem Ecol 5:867–873
Honda K (1980b) Osmeterial secretions of papilionid larvae in the generaLuehdorfia, Graphium and Atrophaneua (Lepidoptera). Insect Biochem 10:583–588
Honda K, Hayashi N (1995) Chemical nature of larval osmeterial secretions of papilionid butterflies in the generaParnassius, Sericinus and Pachliopta. J Chem Ecol 21:859–867
Hsiao TH, Hsiao C, Rothschild M (1980) Characterization of a protein toxin from dried specimens of the garden tiger moth (Arctia caja L.). Toxicon 18:291–299
Huang PK (1980) A study on the bionomic characteristics and control of theBishofia burnet,Histia rhodope Cramer (Lepidoptera, Zygaenidae). J Fujian Agric College 61–79
Jones DA, Parsons J, Rothschild M (1962) Release of HCN from crushed tissues of all stages in the life cycle of species of the Zygaeninae. Nature 193:52–53
Kaiya T, Sakakibara J (1982) Diterpenoids from ericaceous plants. Annu Rep Faculty of Pharmaceutical Sci, Nagoya City Univ 30:1–34
Kettlewell BD (1961) The phenomenon of industrial melanism in Lepidoptera. Annu Rev Entomol 6:245–262
Kim CS, Nishida R, Fukami H, Abe F, Yamauchi T (1994) 14-DeoxyparsonsianidineN-oxide: a pyrrolizidine alkaloid sequestered by the giant danaine butterfly,Idea leuconoe. Biosci Biotech Biochem 58:980–981
Klockars GK, Bowers MD, Cooney B (1993) Leaf variation in iridoid glycoside content ofPlantago laceolata (Plantaginaceae) and oviposition of the buck eye,Junonia coenia (Nymphalidae). Chemoecology 4:72–78
L'Empereur KM, Stermitz FR (1990) Iridoid glycoside metabolism and sequestration byPoladryas minuta (Lepidoptera:Nymphalidae)feeding on Penstimon virgatus (Scrophulariaceae).J Chem Ecol 16: 1495–1506
Mager PP, Seese A, Takeya K (1981) Structure-toxicity relationships applied to grayanotoxins. Pharmazie 36:382–383
Malcolm S, Rothschild M (1983) A danaid mullerian mimic,Euploea core amymone (Cramer) lacking cardenolides in the pupal and adult stage. Biol J Lin Soc 19:27–33
Marsh N, Rothschild M (1974) Aposematic and cryptic Lepidoptera tested on the mouse. J Zool (Lond) 174:89–122
Masutani T, Seyama I, T. Narahashi T, Iwasa J (1981) Strucure-activity relationship for grayanotoxin derivatives in frog skeletal muscle. J Pharmacol Exp Ther 217:812–819
Matsumoto M (1994) 2′-Hydroxy-4′-methoxyacetophenone (paeonol) inExacum affine cv. Biosci Biotech Biochem 58:1892–1893
Meinwald J, Meinwald YC, Wheeler JW, Eisner T, Brower LP (1966) Major components in the exocrine secretion of a male butterfly (Lycorea). Science 151:583–585
Meinwald J, Meinwald YC, Mazzocchi PH (1969) Sex pheromone of the queen butterfly: Chemistry. Science 164:1174–1175
Mix DB, Guinaudeau H, Shamma M (1983) The aristolochic acids and aristolactams. J Nat Prod 45:657–666
Mooe BP, Brown WV, Rothschild M (1990) Methylalkypyrazines in aposematic insects, their host plants and mimics. Chemoecology 1:43–51
Nago H, Matsumoto M (1994) An ecological role of volatiles produced byLasiodiplodia theobromae. Biosci Biotech Biochem 58:1267–1272
Nahrstedt A (1988) Cyanogenesis and the role of cyanogenic compounds in insects. Pp 131–150in Evered D, Harnett S (eds) Cyanide Compounds in Biology. CIBA Symp 140. GB-Chichester: John Wiley & Sons
Nahrstedt A, Davis RH (1986) Uptake of linamarin and lotaustralin from their food-plant by larvae ofZygaena trifolii. Phytochemistry 25:2299–2302
Nahrstedt A, Walther A, Wray V (1982) Sarmentosin epoxide, a new cyanogenic compound fromSedum cepaea. Phytochemistry 21:107–110
Nishida R (1995) Oviposition stimulants of swallowtail butterflies. Pp 17–26in Scriber JM, Tsubaki Y, Lederhous RC (eds) Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Gainesville/FL: Scientific Publishers
Nishida R, Fukami H (1989a). Ecological adaptation of an Aristolochiaceae-feeding swallowtail butterfly,Atrophaneura alcinous, to aristolochic acids. J Chem Ecol 15:2549–2563
Nishida R, Fukami H (1989b) Oviposition stimulants of an Aristolochiaceae-feeding swallowtail butterfly,Atrophaneura alcinous. J Chem Ecol 15:2565–2575
Nishida R, Rothschild M (1995) A cyanoglucoside stored by aSedum-feeding Apollo butterfly,Parnassius phoebus. Experientia 51:267–269
Nishida R, Fukami H, Irie R. Kumazawa Z (1990a) Accumulation of highly toxic ericaceous diterpenoids by the geometrid moth,Arichanna gaschkevitchii. Agric Biol Chem 54:2347–2352
Nishida R, Kim CS, Kawai K, Fukami H (1990b) Methyl hydroxy-benzoates as potent phagostimulants for a male danaid butterfly,Idea leuconoe. Chem Express 5:497–500
Nishida R, Kim CS, Fukami H, Irie R (1991) IdeamineN-oxides: Pyrrolizidine alkaloids sequestered by a danaine butterfly,Idea leuconoe. Agric Biol Chem 55:1787–1797
Nishida R, Weintraub JD, Feeny P, Fukami H (1993) Aristolochic acids fromThottea spp. (Aristolochiaceae) and the osmeterial secretions ofThottea-feeding troidine swallowtail larvae (Papilionidae). J Chem Ecol 19:1587–1594
Nishida R, Rothschild M, Mummery R (1994) A cyanoglucoside, sarmentosin, from the magpie moth,Abraxas grossulariata, Geometridae: Lepidoptera. Phytochemistry 36:37–38
Nishida R, Schulz S, Kim CS, Fukami H, Kuwahara Y, Honda K, Hayashi N (1995) Male pheromone of a giant danaine butterfly,Idea leuconoe. J Chem Ecol: submitted
Pereyra PC, Bowers MD (1988) Iridoid glycosides as oviposition stimulants for the buckeye butterfly,Junonia coenia (Nymphalidae). J Chem Ecol 14:917–928
Pliske TE, Eisner T (1969) Sex pheromone of the queen butterfly: biology. Science 164:1170–1172
Pliske TE, Edgar JA, Culvenor CCJ (1976) The chemical basis of attraction of ithomiine butterflies to plants containing pyrrolizidine alkaloids J Chem Ecol 2:255–262
Poulton EB (1890) The Colour of Animals. 2nd ed. GB-London: Kegan Paul
Reichstein T, Euw Jv, Parsons JA, Rothschild M (1968) Heart poison in the monarch butterfly. Science 161:861–866
Rothschild M (1961) Defensive odours and Müllerian mimicry among insects. Trans R Entomol Soc Lond 113:101–121
Rothschild M (1967) Mimicry, the deceptive way of life. Nat Hist (NY) 76:44–51
Rothschild M (1973) Secondary plant substances and warning coloration in insects. Symp R Entomol Soc Lond 6:59–83
Rothschild M (1979) Mimicry, butterflies and plants. Symb Bot Upsal 22:82–99
Rothschild M, Edgar JA (1978) Pyrrolizidine alkaloids fromSenecio vulgaris sequestered and stored byDanaus plexippus. J Zool (Lond) 186:347–349
Rothschild M, Mummery R (1985) Carotenoids and bile pigments in danaid and swallowtail butterflies. Biol J Linn Soc 24:1–14
Rothschild M, Reichstein RT, Euw Jv, Aplin RT, Harman RRM (1970) Toxic Lepidoptera. Toxicon 8:293–299
Rothschild M, Euw Jv, Reichstein T (1972) Aristolochic acids stored byZerynthia polyxena (Lepidoptera). Insect Biochem 2:334–343
Rothschild M, Aplin RT, Cockrum PA, Edgar JA, Fairweather P, Lees R (1979) Pyrrolizidine alkaloids in arctiid moth with a discussion on host plant relationships and the role of these secondary plant substances in the Arctiidae. Biol J Linn Soc 12:305–326
Rothschild M, Moore BP, Brown WV (1984) Pyrazines as warning odour components in the Monarch butterfly, Danaus plexippus, and in moths of the generaZygaena andAmata (Lepidoptera). Biol J Linn Soc 23:375–380
Rothschild M, Mummery R, Farrell C (1986a) Carotenoids of butterfly models and their mimics (Lep: Papilionidae and Nymphalidea) Biol J Linn Soc 28:359–372
Rothschild M, Nash RJ, Bell EA (1986b) Cycasin in the endangered butterflyEumaeus atala florida. Phyochemistry 25:1853–1854
Sachdev-Gupta K, Feeny PP, Carter M (1993) Oviposition stimulants for the pipevine swallowtail butterfly,Battus philenor (Papilionidea), from anAristolochia host plant: synergism between inositols, aristolochic acids and monogalactosyl diglyceride. Chemoecology 4:19–28
Schneider D, Boppré M, Schneider H, Thompson WR, Boriack CJ, Petty RL, Meinwald J (1975) A pheromone precursor and its uptake in maleDanaus butterflies. J Comp Physiol 97:245–256
Schulz S, Nishida R (1995) Composition of the pheromone system of the male danaine butterfly,Idea leuconoe. Tetrahedron: in press
Schulz S, Francke W, Edgar J, Schneider D (1988) Volatile compounds from androconial organs of danaine and ithomiine butterflies. Z Naturforsch 43c:99–104
Schulz S, Boppré M, Vane-Wright RI (1993) Specific mixture of secretions from male scent organs of African milkweed butterflies (Danainae). Phil Trans R Soc Lond B 342:161–181
Scriber JM, Feeny P (1979) Growth of herbivorous caterpillars in relation to feeding specialization and to the growth form of their food plants. Ecology 60:829–850
Seyama I, Narahashi T (1981) Modulation of sodium channels of squid nerve membranes by grayanotoxin I. J Pharmacol Exp Ther 219:614–624
Siegler DS (1991) Cyanide and cyanogenic glycosides. Pp 35–77in Rosenthal GA, Janzen DH (eds) Herbivores: Their Interactions with Secondary Plant Metabolites. Vol I: The Chemical Participants. 2nd ed. New York: Academic Press
Stermitz FR, Gardner DR, Odendaal FJ, Ehrlich PR (1986)Euphydryas anicia (Lepidoptera: Nymphalidae) utilization of iridoid glycosides fromCastilleja andBesseya (Scrophulariaceae) host plants. J Chem Ecol 12:1459–1468
Stermitz FR, Gardner DR, McFarland N (1988) Iridoid glycoside sequestration by two aposematicPenstimon-feeding geometrid larvae. J Chem Ecol 14:435–441
Trigo JR, Brown KS (1990) Variation of pyrrolizidine alkaloids in Ithomiinae: a comparative study between species feeding on Apocynaceae and Solanaceae. Chemoecology 1:22–29
Uesugi K (1995) Mimicry inPapilio polytes and its ecological meaning. Pp 165–172in Scriber JM, Tsubaki Y, Lederhous RC (eds) Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Gainesville/FL: Scientific Publ
Urzúa A, Priestap H (1985) Aristolochic acids fromBattus polydamas. Biochem Syst Ecol 13:169–170
Urzúa A, Salgado G, Gassels BK, Eckhardt G (1983) Aristolochic acids inAristolochia chilensis and theAristolochia-feeder,Battus archidamas (Lepidoptera). Collect Czech Chem Commun 48:1513–1519
Verschaffelt E (1811) The cause determining the selection of food in some herbivorous insects. Proc Acad Sci Amsterdam 13:536–542
Witthohn K, Naumann CM (1987) Cyanogenesis — a general phenomenon in the Lepidoptera? J Chem Ecol 13:1789–1809
Wray V, David RH, Nahrstedt A (1983) Biosynthesis of cyanogenic glycosides in butterflies and moths: incorporation of valine and isoleucine into linamarin and lotaustralin byZygaena andHeliconius species (Lepidoptera). Z Naturforsch 38c:583–588
Zushi S, Miyagawa J, Yamamoto M, Kataoka K, Seyama I (1993) Effect of grayanotoxin on the frog neuromuscular junction. J Pharmacol Exp Ther 226:269–275
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nishida, R. Sequestration of plant secondary compounds by butterflies and moths. Chemoecology 5, 127–138 (1994). https://doi.org/10.1007/BF01240597
Issue Date:
DOI: https://doi.org/10.1007/BF01240597