Summary
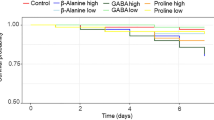
We investigated the role of the iridoid glycoside, catalpol, as a deterrent to the predator,Camponotus floridanus. Four laboratory colonies of this ant were offered buckeye caterpillars (Junonia coenia: Nymphalidae) raised on diets with and without catalpol. The same colonies were offered sugar-water solutions containing varying concentrations of catalpol, in both no-choice and choice tests. Regardless of diet, buckeye caterpillars appeared to be morphologically protected from predation by the ants, possibly because of their large spines or tough cuticle. However, buckeyes raised on diets with catalpol had high concentrations of catalpol in their hemolymph; extracts of this high-catalpol hemolymph proved to be an effective deterrent to the ants. When starved ants were not given the choice of food items, they were more likely to consume sucrose solutions that contained 5 mg catalpol/ml or 10 mg catalpol/ml than they were to consume solutions with 20 mg catalpol/ml. When they were given a choice of sugar solution or a sugar solution containing catalpol, the ants avoided solutions with catalpol at any of these concentrations. Ant colony responses to catalpol in sucrose solutions varied considerably over time and among colonies.
Similar content being viewed by others
References
Bernays EA (1988) Host specificity in phytophagous insects: selection pressure from generalist predators. Entomol exp appl 49:131–140
Bobbitt JM, Segebarth DP (1969) Iridoid glycosides and similar substances. Pp 1–145in Taylor WI, Battersby AR (eds) Cyclopentanoid Terpene Derivatives. New York: Academic Press
Bowers MD (1980) Unpalatability as a defense strategy ofEuphydryas phaeton. Evolution 34:586–600
Bowers MD (1984) Iridoid glycosides and hostplant specificity in larvae of the buckeye butterfly,Junoni coenia (Nymphalidae). J Chem Ecol 10:1567–1577
Bowers MD (1991) Iridoid glycosides. Pp 297–325in Rosenthal G, Berenbaum M (eds) Herbivores: Their Interactions with Secondary Plant Metabolites. 2nd ed. Volume I: The Chemical Participants. Orlando FL: Academic Press
Bowers MD, Collinge SK (1992) Fate of iridoid glycosides in different life stages of the buckeye,Junonia coenia (Lepidoptera: Nymphalidae). J Chem Ecol 18:317–831
Bowers MD, Puttick GM (1989) Iridoid glycosides and insect feeding preferences: gypsy moths (Lymantria dispar, Lymantriidae) and buckeyes (Junonia coenia, Nymphalidae). Ecol Entomol 14:247–256
Bowers MD, Stamp NE (1992) Chemical variation within and between individuals ofPlantago lanceolata (Plantaginaceae). J Chem Ecol 18:985–995
Brower LP (1984) Chemical defense in butterflies. Pp 109–134in Vane-Wright RI, Ackery PR (eds) The Biology of Butterflies. Symp Royal Entomol Soc 11. New York: Academic Press
Brower LP, Brower JVZ, Corvino JM (1967) Plant poisons in a terrestrial food chain. Proc Nat Acad Sci 57:893–898
Campbell RW, Torgersen TR, Srivastava N (1983) A suggested role for predaceous birds and ants in the population dynamics of the western spruce budworm. Forest Science 29:779–790
Carlin NF, Schwartz PH (1989) Pre-imaginal experience and nestmate brood recognition in the carpenter ant,Camponotus floridanus. Anim Behav 38:89–95
Carney WP (1970) Laboratory maintenance of carpenter ants. Ann Entomol Soc Am 63:333–334
Cott HB (1940) Adaptive Coloration in Animals. New York: Oxford University Press
Dejean A (1988) Prey capture byCamponotus maculatus (Formicidae — Formicinae). Biol Behav 13:97–115
Dempster JP (1984) The natural enemies of butterflies. Pp 97–104in Vane-Wright RI, Ackery PR (eds) The Biology of Butterflies. Symp Royal Entomol Soc 11. New York: Academic Press
Duff R, Bacon J, Mucndie C, Farmer V, Russell J, Forrester J, Forrester A (1965) Catalpol and methylcatalpol: naturally occurring glycosides inPlantago andBuddleia species. Biochem J 96:1–5
Edmunds M (1974) Defence in Animals. GB-Essex: Longman
Ford EB (1945) Butterflies. London: Collins
Gardner DR, Stermitz FR (1988) Hostplant utilization and iridoid glycoside sequestration byEuphydryas anicia individuals and populations. J Chem Ecol 15:2147–2168
Inouye H (1991) Iridoids. Pp 99–143in Charlwood BV, Banthorpe DV (eds) Methods in Plant Biochemistry. Vol 7: Terpenoids. San Diego: Academic Press
Jenson SR (1991) Plant iridoids, their biosynthesis and distribution in angiosperms. Pp 133–158in Harborne JB, Tomas-Barberan FA (eds) Ecological Chemistry and Biochemistry of Plant Terpenoids. Oxford: Clarenden Press
Lanza J (1991) Response of fire ants (Formicidae:Solenopsis invicta andS. geminata) to artificial nectars with amino acids. Ecol Entomol 16:203–210
Mason RR, Trogersen TR (1983) Mortality of larvae in stocked cohorts of the douglas-fir tussock moth,Orgyia pseudotsugata (Lepidoptera: Lymantriidae). Can Entomol 115:1119–1127
Merck Index, Tenth Edition (1983) Windholz M, Budavari S, Blumetti RF, Otterbein ES (eds). New Jersey: Merck & Co
Morris RF (1972) Predation by wasps, birds, and mammals onHyphantria cunea. Can Entomol 104:1581–1591
Nonacs P (1991) Less growth with more food: how insect-prey availability changes colony demographics in the ant,Camponotus floridanus. J Insect Physiol 37:891–898
Peterson CH, Renaud PE (1989) Analysis of feeding preference experiments. Oecologia 80:82–86
Puttick GM, Bowers MD (1988) Effect of quantitative variation in allelochemicals on a generalist insect: iridoid glycosides and the Southern armyworm. J Chem Ecol 14:335–351
Rothschild M (1985) British aposematic Lepidoptera. Pp 9–62in Heath J, Emmet AM (eds) The Moths and Butterflies of Great Britain and Ireland. GB-Essex: Harley Books
Stamp NE, Bowers MD (1988) Direct and indirect effects of predatory wasps (Polistes sp: Vespidae) on gregarious caterpillars (Hemileuca lucina: Saturniidae). Oecologia 75:619–624
Steward VB, Smith KG, Stephen FM (1988) Predation by wasps on lepidopteran larvae in an Ozark forest canopy. Ecol Entomol 13:81–86
Youngs LC (1983) Predaceous ants in biological control of insect pests of a North American forest. Bull Entomol Soc Am 29: 47–50
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de la Fuente, MA., Dyer, L.A. & Bowers, M.D. The iridoid glycoside, catalpol, as a deterrent to the predatorCamponotus floridanus (Formicidae). Chemoecology 5, 13–18 (1994). https://doi.org/10.1007/BF01259968
Issue Date:
DOI: https://doi.org/10.1007/BF01259968