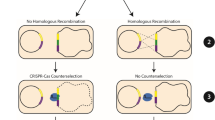
Abstract
Clostridium includes a number of species, such as thermophilic Clostridium thermocellum and mesophilic Clostridium cellulolyticum, producing biofuels and chemicals from lignocellulose, while genetic engineering is necessary to improve wild-type strains to fulfill the requirement of industrialization. ClosTron system is widely used in the gene targeting of Clostridium because of its high efficiency and operability. However, the targetron plasmid present in cell hinders the successive gene disruption. To solve this problem, a pyrF-based screening system was developed and implemented in C. cellulolyticum strain H10 in this study for efficient targetron plasmid curing. The screening system was composed of a pyrF-deleted cell chassis (H10ΔpyrF) constructed via homologous recombination and a PyrF expression cassette located in a targetron plasmid containing an erythromycin resistance gene. With the screening system, the gene targeting could be achieved following a two-step procedure, including the first step of gene disruption through targetron transformation and erythromycin selection and the second step of plasmid curing by screening with 5-fluoroorotic acid. To test the developed screening system, successive inactivation of the major cellulosomal exocellulase Cel48F and the scaffoldin protein CipC was achieved in C. cellulolyticum, and the efficient plasmid curing was confirmed. With the assistance of the pyrF-based screening system, the targetron plasmid-cured colonies can be rapidly selected by one-plate screening instead of traditional days' unguaranteed screening, and the successive gene disruption becomes accomplishable with ClosTron system with improved stability and efficiency, which may promote the metabolic engineering of Clostridium species aiming at enhanced production of biofuels and chemicals.



Similar content being viewed by others
References
Abdou L, Boileau C, de Philip P, Pages S, Fierobe HP, Tardif C (2008) Transcriptional regulation of the Clostridium cellulolyticum cip-cel operon: a complex mechanism involving a catabolite-responsive element. J Bacteriol 190:1499–1506
Berzin V, Kiriukhin M, Tyurin M (2013) “Curing” of plasmid DNA in acetogen using microwave or applying an electric pulse improves cell growth and metabolite production as compared to the plasmid-harboring strain. Arch Microbiol 195:181–188
Boeke JD, LaCroute F, Fink GR (1984) A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet 197:345–346
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254
Brana H, Benada O, Navratil O, Cejka K, Hubacek J (1983) Stability of the hybrid plasmid pIM138 and its curing by some eliminating agents. Folia Microbiol (Praha) 28:441–445
Chen Y, McClane BA, Fisher DJ, Rood JI, Gupta P (2005) Construction of an alpha toxin gene knockout mutant of Clostridium perfringens type A by use of a mobile group II intron. Appl Environ Microbiol 71:7542–7547
Chung D, Farkas J, Westpheling J (2013) Overcoming restriction as a barrier to DNA transformation in Caldicellulosiruptor species results in efficient marker replacement. Biotechnol Biofuels 6:82
Cui GZ, Hong W, Zhang J, Li WL, Feng Y, Liu YJ, Cui Q (2012) Targeted gene engineering in Clostridium cellulolyticum H10 without methylation. J Microbiol Methods 89:201–208
Desvaux M (2005) Clostridium cellulolyticum: model organism of mesophilic cellulolytic clostridia. FEMS Microbiol Rev 29:741–764
Desvaux M, Guedon E, Petitdemange H (2000) Cellulose metabolism by Clostridium cellulolyticum growing in batch culture on defined medium. Appl Environ Microbiol 66:2461–2470
Dong H, Zhang Y, Dai Z, Li Y (2010) Engineering Clostridium strain to accept unmethylated DNA. PLoS ONE 5:e9038
Enyeart PJ, Chirieleison SM, Dao MN, Perutka J, Quandt EM, Yao J, Whitt JT, Keatinge-Clay AT, Lambowitz AM, Ellington AD (2013) Generalized bacterial genome editing using mobile group II introns and Cre-lox. Mol Syst Biol 9:685
Frazier CL, San Filippo J, Lambowitz AM, Mills DA (2003) Genetic manipulation of Lactococcus lactis by using targeted group II introns: generation of stable insertions without selection. Appl Environ Microbiol 69:1121–1128
Guedon E, Desvaux M, Petitdemange H (2002) Improvement of cellulolytic properties of Clostridium cellulolyticum by metabolic engineering. Appl Environ Microb 68:53–58
Heap JT, Ehsaan M, Cooksley CM, Ng YK, Cartman ST, Winzer K, Minton NP (2012) Integration of DNA into bacterial chromosomes from plasmids without a counter-selection marker. Nucleic Acids Res 40:e59
Heap JT, Kuehne SA, Ehsaan M, Cartman ST, Cooksley CM, Scott JC, Minton NP (2010) The ClosTron: mutagenesis in Clostridium refined and streamlined. J Microbiol Methods 80:49–55
Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP (2007) The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods 70:452–464
Higashide W, Li Y, Yang Y, Liao JC (2011) Metabolic engineering of Clostridium cellulolyticum for isobutanol production from cellulose. Appl Environ Microbiol 77:2727–2733
Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR (1989) Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68
Hovi M, Sukupolvi S, Edwards MF, Rhen M (1988) Plasmid-associated virulence of Salmonella enteritidis. Microb Pathog 4:385–391
Jennert KC, Tardif C, Young DI, Young M (2000) Gene transfer to Clostridium cellulolyticum ATCC 35319. Microbiology 146:3071–3080
Jia K, Zhu Y, Zhang Y, Li Y (2011) Group II intron-anchored gene deletion in Clostridium. PLoS ONE 6(1):e16693
Karberg M, Guo H, Zhong J, Coon R, Perutka J, Lambowitz AM (2001) Group II introns as controllable gene targeting vectors for genetic manipulation of bacteria. Nat Biotech 19:1162–1167
Kuehne SA, Heap JT, Cooksley CM, Cartman ST, Minton NP (2011) ClosTron-mediated engineering of Clostridium. Methods Mol Biol 765:389–407
Lambowitz AM, Zimmerly S (2011) Group II introns: mobile ribozymes that invade DNA. Cold Spring Harb Perspect Biol 3(8):a003616
Li Y, Tschaplinski TJ, Engle NL, Hamilton CY, Rodriguez M Jr, Liao JC, Schadt CW, Guss AM, Yang Y, Graham DE (2012) Combined inactivation of the Clostridium cellulolyticum lactate and malate dehydrogenase genes substantially increases ethanol yield from cellulose and switchgrass fermentations. Biotechnol Biofuels 5:2
Lin L, Song H, Ji Y, He Z, Pu Y, Zhou J, Xu J (2010) Ultrasound-mediated DNA transformation in thermophilic gram-positive anaerobes. PLoS ONE 5:e12582
Liu H, Han J, Liu X, Zhou J, Xiang H (2011) Development of pyrF-based gene knockout systems for genome-wide manipulation of the archaea Haloferax mediterranei and Haloarcula hispanica. J Genet Genomics 38:261–269
Lynd LR, van Zyl WH, McBride JE, Laser M (2005) Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol 16:577–583
Maamar H, Abdou L, Boileau C, Valette O, Tardif C (2006) Transcriptional analysis of the cip-cel gene cluster from Clostridium cellulolyticum. J Bacteriol 188:2614–2624
Maamar H, Valette O, Fierobe HP, Belaich A, Belaich JP, Tardif C (2004) Cellulolysis is severely affected in Clostridium cellulolyticum strain cipCMut1. Mol Microbiol 51:589–598
Olson DG, Tripathi SA, Giannone RJ, Lo J, Caiazza NC, Hogsett DA, Hettich RL, Guss AM, Dubrovsky G, Lynd LR (2010) Deletion of the Cel48S cellulase from Clostridium thermocellum. Proc Natl Acad Sci U S A 107:17727–17732
Pavlostathis SG, Miller TL, Wolin MJ (1988) Fermentation of insoluble cellulose by continuous cultures of Ruminococcus albus. Appl Environ Microb 54:2655–2659
Perret S, Maamar H, Belaich JP, Tardif C (2004) Use of antisense RNA to modify the composition of cellulosomes produced by Clostridium cellulolyticum. Mol Microbiol 51:599–607
Rattanachaikunsopon P, Phumkhachorn P (2009) Glass bead transformation method for Gram-positive bacteria. Braz J Microbiol 40:923–926
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sato T, Fukui T, Atomi H, Imanaka T (2005) Improved and versatile transformation system allowing multiple genetic manipulations of the hyperthermophilic archaeon Thermococcus kodakaraensis. Appl Environ Microbiol 71:3889–3899
Shao L, Hu S, Yang Y, Gu Y, Chen J, Jiang W, Yang S (2007) Targeted gene disruption by use of a group II intron (targetron) vector in Clostridium acetobutylicum. Cell Res 17:963–965
Spengler G, Molnar A, Schelz Z, Amaral L, Sharples D, Molnar J (2006) The mechanism of plasmid curing in bacteria. Curr Drug Targets 7:823–841
Suzuki H, Murakami A, Yoshida K (2012) Counterselection system for Geobacillus kaustophilus HTA426 through disruption of pyrF and pyrR. Appl Environ Microbiol 78:7376–7383
Svetlitchnyi VA, Kensch O, Falkenhan DA, Korseska SG, Lippert N, Prinz M, Sassi J, Schickor A, Curvers S (2013) Single-step ethanol production from lignocellulose using novel extremely thermophilic bacteria. Biotechnol Biofuels 6:31
Tardif C, Maamar H, Balfin M, Belaich JP (2001) Electrotransformation studies in Clostridium cellulolyticum. J Ind Microbiol Biot 27:271–274
Tripathi SA, Olson DG, Argyros DA, Miller BB, Barrett TF, Murphy DM, McCool JD, Warner AK, Rajgarhia VB, Lynd LR, Hogsett DA, Caiazza NC (2010) Development of pyrF-based genetic system for targeted gene deletion in Clostridium thermocellum and creation of a pta mutant. Appl Environ Microbiol 76:6591–6599
Uhlin BE, Nordstrom K (1975) Plasmid incompatibility and control of replication: copy mutants of the R-factor R1 in Escherichia coli K-12. J Bacteriol 124:641–649
Acknowledgments
We thank Dr. Weihong Jiang and Dr. Sheng Yang from the Institute of Plant Physiology and Ecology, Shanghai, People's Republic of China for providing plasmid pSY6. We thank Dr. Yin Li and Dr. Hongjun Dong from the Institute of Microbiology, Chinese Academy of Sciences, Beijing, People's Republic of China for the helpful discussion. This work was supported by the National Basic Research Program of China (973 Program, grant 2011CB707404), the Key Technologies R&D Program from the Ministry of Science and Technology of China (grant 2011BAD22B02), and the Instrument Developing Project of the Chinese Academy of Sciences (grant no. YZ201138).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 233 kb)
Rights and permissions
About this article
Cite this article
Cui, GZ., Zhang, J., Hong, W. et al. Improvement of ClosTron for successive gene disruption in Clostridium cellulolyticum using a pyrF-based screening system. Appl Microbiol Biotechnol 98, 313–323 (2014). https://doi.org/10.1007/s00253-013-5330-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5330-y