Summary
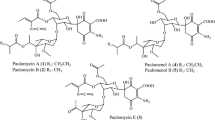
Streptomyces viridochromogenes Tü494 produces the antibiotic phosphinothricyl-alanyl-alanine (Ptt). Ptt-non-producing mutants were isolated following N-methyl-N′-nitro-N-nitrosoguanidine (NTG) or UV light treatment of spore suspensions. In co-synthesis and bioconversion experiments the mutational blocks in the biosynthetic pathway were localized. The mutant NTG1 was analysed in detail. This mutant acts as a secretor for all other mutants. From bioconversion experiments with presumptive precursors circumstantial evidence was obtained that NTG1 is mutated in a gene involved in the alanylation of N-acetyl-demethyl-phosphinothricin. Using a cosmid gene library the DNA region complementing the defective gene of mutant NTG1 was isolated on a 4-kb BamHI fragment. Subcloning experiments showed that a 3-kb BglII/BamHI fragment is sufficient for complementation of mutant NTG1.
Similar content being viewed by others
References
Baltz RH, Stonesifer J (1985) Phenotypic changes associated with loss of expression of tylosin biosynthesis and resistance genes in Streptomyces fradiae. J Antibiot 38:1226–1236.
Bayer E, Gugel KH, Hagenmeier H, Jessipow S, König WA, Zähner H (1972) Stoffwechselprodukte von Mikroorganismen. Phosphinothricin und Phosphinothricyl-alanyl-alanin. Helv Chim Acta 55:224–239.
Bibb MJ, Ward JM, Hopwood DA (1978) Transformation of plasmid DNA into Streptomyces at high frequencies. Nature 274:398–400.
Chater KF, Hopwood DA, Kieser T, Thompson CJ (1982) Gene cloning in Streptomyces. Curr Top Microbiol Immunol 96:69–95.
Clarke CH, Hopwood DA (1976) Ultraviolet mutagenesis in Streptomyces coelicolor: induction of reversions in a polyauxotrophic strain. Mutat Res 41:201–208.
Cohen SN, Chang ACY, Hsu L (1972) Non chromosomal antibiotic resistance in bacteria: genetic transformation of E. coli by R-factor DNA. Proc Natl Acad Sci USA 69:2110–2114.
Cox KL (1986) The use of recombinant DNA techniques to study tylosin biosynthesis and resistance in Streptomyces fradiae. J Nat Prod 49:971–980.
Delic V, Pigac J, Sermonti G (1969) Detection and study of cosynthesis of tetracycline antibiotics by an agar method. J Gen Microbiol 55:103–108.
Delic V, Hopwood DA, Friend EJ (1970) Mutagenesis by N-methyl-N′-nitro-N-nitrosoguanidine (NTG) in Streptomyces coelicolor. Mutat Res 9:167–182.
Diddens H, Zähner H, Kraas E, Göhring W, Jung G (1976) On the transport of tripeptide antibiotics in bacteria. Eur J Biochem 66:11–23.
Furumai T, Takeda K, Okanishi M (1982) Function of plasmids in the production of aureothricin. I. Elimination of plasmids and alteration of phenotypes caused by protoplast regeneration in Streptomyces kasugaensis. J Antibiot 35:1367–1373.
Grunstein M, Hogness DS (1975) Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA 72:3961–3965.
Hara O, Anzai H, Imai S, Kumada Y, Murakami T, Itoh R, Takano E, Satoh A, Nagaoka K (1988) The bialaphos biosynthetic genes of Streptomyces hygroscopicus: cloning and analysis of the genes involved in the alanylation step. J Antibiot 41:538–547.
Hohn B, Collins J (1980) A small cosmid for efficient cloning of large DNA fragments. Gene 11:291–298.
Holmes DS, Quigley M (1981) A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem 114:193–197.
Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, ward JM, Schrempf H (1985) Genetic manipulation of Streptomyces: a laboratory manual. John Innes Foundation, Norwich.
Ichikawa T, Date M, Ishikura T, Ozaki S (1971) Improvement of kasugamycin-producing strains by the agar piece method and prototroph method. Folia Microbiol 16:218–224.
Imai S, Seto H, Sasaki T, Tsuruoka T, Ogawa H, Satoh A, Inouye S, Niida T, Otake N (1985) Studies on the biosynthesis of bialaphos (SF-1293). 6. Production of N-acetyl-demethylphosphinothricin and N-acetyl-bialaphos by blocked mutants of Streptomyces hygroscopicus SF-1293 and their roles in the biosynthesis of bialaphos. J Antibiot 38:687–690.
Kieser T (1984) Factors affecting the isolation of ccc DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:223–238.
Kondo Y, Shomura T, Ogawa Y, Tsuruoka T, Watanabe H, Totsukawa K, Suzuki T, Moriyama C, Yoshida J, Inouye I, Niida T (1973) Studies on a new antibiotic SF-1293. I. Isolation and physico-chemical and biological characterization of SF-1293 substances. Sci Rep Meiyi Seika Kaisha 13:34–41.
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N. Y.
Murakami T, Anzai H, Imai S, Satoh A, Nagaoka K, thompson CJ (1986) The bialaphos biosynthetic genes of Streptomyces hygroscopicus: molecular cloning and characterization of the gene cluster. Mol Gen Genet 205:42–50.
Muth G, Nussbaumer B, Wohlleben W, Pühler A (1989) A vector system with temperature-sensitive replication for gene disruption and mutational cloning in streptomycetes. Mol Gen Genet 219:341–348.
Okanishi M, Suzuki K, Umezawa H (1974) Formation and reversion of streptomycete protoplasts: cultural conditions and morphological study. J Gen Microbiol 80:389–400.
Rhodes PM, Winskill N, Friend EJ, Warren M (1981) Biochemical and genetic characterization of Streptomyces rimosus impaired in oxytetracycline biosynthesis. J Gen Microbiol 124:329–338.
Seto H, Imai S, Tsuruoka T, Ogawa H, Satoh A, Sasaki T, Otake N (1983) Studies on the biosynthesis of bialaphos (SF-1293). III. Production of phosphonic acid derivatives MP-103, MP-104 and MP-105 by a blocked mutant of Streptomyces hygroscopicus SF1293 and their roles in the biosynthesis of bialaphos. Biochem Biophys Res Commun 111:1008–1014.
Ward JM, Janssen GR, Buttner MJ, Bibb MJ (1986) Construction and characterization of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet 203:468–478.
Weber JH, Wierman CK, Hutchinson CR (1985) Genetic analysis of erythromycin production in Streptomyces erythreus. J Bacteriol 164:425–433.
Wohlleben W, Muth G, Birr E, Pühler A (1986) A vector system for cloning in Streptomyces and Escherichia coli. In: Szabo G, Biro S, Goodfellow M (eds) Sixth International Symposium on Actinomycetes Biology. Akademiai Kiado, Budapest, pp 99–101.
Wohlleben W, Arnold W, Broer I, Hillemann D, Strauch E, Pühler A (1988) Nucleotide sequence of the phosphinothricin N-acetyltransferase gene from Streptomyces viridochromogenes Tü494 and its expression in Nicotiana tabacum. Gene 70:25–37.
Author information
Authors and Affiliations
Additional information
Formerly Susanne Müller
Offprint requests to: W. Wohlleben
Rights and permissions
About this article
Cite this article
Alijah, R., Dorendorf, J., Talay, S. et al. Genetic analysis of the phosphinothricin-tripeptide biosynthetic pathway of Streptomyces viridochromogenes Tü494. Appl Microbiol Biotechnol 34, 749–755 (1991). https://doi.org/10.1007/BF00169345
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00169345