Abstract
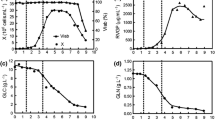
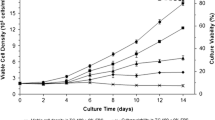
The transmembrane rabies virus glycoprotein (RVGP) is the main antigen of vaccine formulations used around the world to prevent rabies, the most lethal preventable infectious disease known. The objective of this work was to evaluate the potential of a bioreactor using wave-induced agitation in the initial steps of scaling up the rRVGP production process by a Drosophila melanogaster S2 cell line to produce rRVGP in sufficient quantities for immunization and characterization studies. Taking advantage of some remarkable features recognized in Drosophila S2 cells for scaling the culture process, a robust recombinant lineage (S2MtRVGPH-His) engineered by our group for the expression of rRVGP using a copper-inducible promoter was used in the bioreactor cultures. The WAVE Bioreactor was chosen because it represents an innovative approach to the cultivation of animal cells using single-use technology. For that purpose, we firstly established a procedure for culturing the S2MtRVGPH-His lineage in 100 mL Schott flasks. Using an inoculum of 5 × 105 cells/mL in culture medium (Sf900-III) induced with solution of CuSO4 (0.7 mM) and a convenient pH range (6.2–7.0), optimal parameter values such as time of induction (72 h) and temperature (28 °C) to increase rRVGP production could be defined. This procedure was reproduced in culture experiments conducted in a WAVE Bioreactor™ 2/10 using a 2 L Cellbag. The results in Schott flasks and in WAVE Bioreactor™ were very similar, yielding a maximum titer of rRVGP above of 1 mg.L−1. The immunization study showed that the rRVGP produced in the bioreactor was of high immunogenic quality.



Similar content being viewed by others
References
Astray RM, Augusto E, Yokomizo AY, Pereira CA (2008) Analytical approach for the extraction of recombinant membrane viral glycoprotein from stably transfected Drosophila melanogaster cells. Biotechnol J 3:98–103. https://doi.org/10.1002/biot.200700179
Astray RM, Jorge SAC, Lemos MAN, Yokomizo AY, Boldorini VLL, Puglia ALP, Ribeiro OG, Pereira CA (2013) Kinetic studies of recombinant rabies virus glycoprotein (RVGP) cDNA transcription and mRNA translation in Drosophila melanogaster S2 cell populations. Cytotechnology 65:829–838
Astray RM, Jorge SCA, Pereira CA (2017) Rabies vaccine development by expression of recombinant viral glycoprotein. Arch Virol 162:323–332. https://doi.org/10.1007/s00705-016-3128-9
Barnes LM, Bentley CM, Dickson AJ (2001) Characterization of the stability of recombinant protein production in the GS-NS0 expression system. Biotechnol Bioeng 73:261–270
Bathia R, Jesionowski G, Ferrance J, Ataai MM (1997) Insect cell physiology. Cytotechnology 24:1–9
Borash DJ, Pierce VA, Gibbs AG, Mueller LD (2000) Evolution of ammonia and urea tolerance in Drosophila melanogaster: resistance and cross-tolerance. J Insect Physiol 46:763–769
Bovo R, Galesi ALL, Jorge SAC, Piccoli RAM, Moraes AM, Pereira CA, Augusto EFP (2008) Kinetic response of a Drosophila melanogaster cell line to different medium formulations and culture conditions. Citotechnology 57:23–35. https://doi.org/10.1007/s10616-008-9146-z
Butler M (2005) Animal cell cultures: recent achievements and perspectives in the production of biopharmaceuticals. Appl Microbiol Biotechnol 68:283–291. https://doi.org/10.1007/s00253-005-1980-8
Clements DE, Coller BG, Lieberman MM, Ogata S, Wang G, Harada KE, Putnak JR, Ivy JM, Mcdonell M, Gary S, Peters ID, Leung J, Weeks-levy C, Nakano ET, Humphreys T (2011) Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine 28:2705–2715. https://doi.org/10.1016/j.vaccine.2010.01.022.Development
Doyle A, Griffths J (2007) Cell and tissue culture: laboratory procedures in biotechnology. Wiley, Chichester
Ertl HCJ (2009) Novel vaccines to human rabies. PLoS Negl Trop Dis 3:e515. https://doi.org/10.1371/journal.pntd.0000515
Fries S, Glazomitsky K, Woods A, Forrest G, Hsu A, Olewinski R, Robinson D, Chartrain M (2005) Evaluation of disposable bioreactors: rapid production of recombinant proteins by several animal cell lines. Bioprocess Int 3(Supp6):36–44
Galesi ALL, Aguiar MA, Astray RM, Augusto EFP, Moraes ÂM (2008) Growth of recombinant Drosophila melanogaster Schneider 2 cells producing rabies virus glycoprotein in bioreactor employing serum-free medium. Cytotechnology 57:73–81. https://doi.org/10.1007/s10616-008-9139-y
Geisler C, Mabashi-Asazuma H, Jarvis D (2015) An overview and history of glyco-engineering in insect expression systems. Methods Mol Biol 1321:131–152
Glick B (1995) Metabolic load and heterologous gene expression. Biotechnol Adv 13:247–261
Gòdia F, Cairo J (2006) Cell Metabolism. In: Ozturk S, Hu W (eds) Cell culture technology for pharmaceutical and cell-based therapies. Taylor&Francis Group, Pennsylvania, pp 81–112
Hayter PM, Kirkby NF, Spier RE (1992) Relationship between hybridoma growth and monoclonal antibody production. Enzym Microb Technol 14:454–461
Heinrikson RL, Meredith SC (1984) Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem 136:65–74
Lemos MAN, Santos AS, Astray RM, Pereira CA, Jorge SAC (2009) Rabies virus glycoprotein expression in Drosophila S2 cells. I: design of expression/selection vectors, subpopulations selection and influence of sodium butyrate and culture medium on protein expression. J Biotechnol 143:103–110. https://doi.org/10.1016/j.jbiotec.2009.07.003
Lim HJ, Cha HJ (2006) Observation and modeling of induction effect on human transferrin production from stably transfected Drosophila S2 cell culture. Enzym Microb Technol 39:208–214. https://doi.org/10.1016/j.enzmictec.2005.10.021
Loffelhotlz C, Kaiser SC, Kraume M, Eibl R, Dieter E (2013) Dynamic single-use bioreactors used in modern liter- and m3 - scale biotechnological processes: engineering characteristics and scaling up. Adv Biochem Eng Biotechnol 138:1–44. https://doi.org/10.1007/10_2013_187
Macaranga L, Cruz PE, Aunins JG, Carrondo MJ (2002) Production of Core and virus-like particles with Baculovirus infected insect cells. In: Scheper T (ed) Advanced in biochemical engineering biotechnology: tools and applications of biochemical engineering science. Springer-Verlag, Berlin, pp 183–207
Moraes ÂM, Jorge SAC, Astray RM, Suazo CAT, Calderón Riquelme CE, Augusto EFP, Tonso A, Pamboukian MM, Piccoli RAM, Barral MF, Pereira CA (2012) Drosophila melanogaster S2 cells for expression of heterologous genes: from gene cloning to bioprocess development. Biotechnol Adv 30:613–628. https://doi.org/10.1016/j.biotechadv.2011.10.009
Norgate M, Southon A, Greenough M, Cater M, Farlow A, Bush AI, Subramaniam VN, Burke R, Camakaris J (2010) Syntaxin 5 is required for copper homeostasis in Drosophila and Mammals. PLoS One 5:e14303. https://doi.org/10.1371/journal.pone.0014303
Perrin P, Thibodeau L, Sureau P (1985) Rabies immunosome (subunit vaccine) structure and immunogenicity. Pre- and post-exposure protection studies. Vaccine 3:325–332. https://doi.org/10.1016/S0264-410X(85)90224-5
Perrin P, Lafon M, Sureau P (1996) Enzyme linked immunosorbent assay (ELISA) for the determination of glycoprotein content of rabies vaccines. MeslinFX, Kaplan MM, Koprowski H Lab Tech rabies WHO, Geneva, pp 383–388
Portner R, Schafer T (1996) Modelling hybridoma cell growth and metabolism—a comparison of selected models and data. Biotechnol J 49:119–135
Roche S, Gaudin Y (2002) Characterization of the equilibrium between the native and fusion-inactive conformation of rabies virus glycoprotein indicates that the fusion complex is made of several trimers. Virology 297:128–135. https://doi.org/10.1006/viro.2002.1429
Rossi N, Silva BG, Astray R, Swiech K, Pereira CA, Suazo CAT (2012) Effect of hypothermic temperatures on production of rabies virus glycoprotein by recombinant Drosophila melanogaster S2 cells cultured in suspension. J Biotechnol 161:328–335. https://doi.org/10.1016/j.jbiotec.2012.05.016
Santos N, Rocca M, Pereira CA, Ventini DC, Puglia ALP, Jorge SAC, Lemos MAN, Astray RM (2016) Impact of recombinant Drosophila S2 cell population enrichment on expression of rabies virus glycoprotein. Cytotechnology 68:2605–2611
Scaraffia PY, Wells MA (2003) Proline can be utilized as an energy substrate during flight of Aedes aegypti females. J Insect Physiol 49:591–601. https://doi.org/10.1016/S0022-1910(03)00031-3
Singh V (1999) Disposable bioreactor for cell culture using wave-induced agitation. Cytotechnology 30(1–3):149–158
Swiech K, Rossi N, Silva BG, Jorge SAC, Astray RM, Suazo CAT (2008a) Bioreactor culture of recombinant Drosophila melanogaster S2 cells: characterization of metabolic features related to cell growth and production of the rabies virus glycoprotein. Cytotechnology 57:61–66
Swiech K, Silva C, Arantes MK, Santos AS, Astray RM, Pereira CA, Suazo AT (2008b) Characterization of growth and metabolism of Drosophila melanogaster cells transfected with the rabies-virus glycoprotein gene. Biotechnol Appl Biochem 49:41–49. https://doi.org/10.1042/BA20060148
Ventini DC, Astray RM, Lemos MAN, Jorge SAC, Riquelme CC, Suazo CAT, Tonso A, Pereira CA (2010) Recombinant rabies virus glycoprotein synthesis in bioreactor by transfected Drosophila melanogaster S2 cells carrying a constitutive or an inducible promoter. J Biotechnol 146:169–172. https://doi.org/10.1016/j.jbiotec.2010.02.011
Wang L, Hu H, Yang J, Wang F, Kaisermayer C, Zhou P (2012) High yield of human monoclonal antibody produced by stably transfected Drosophila Schneider 2 cells in perfusion culture using wave bioreactor. Mol Biotechnol 52:170–179. https://doi.org/10.1007/s12033-011-9484-5
White JA, Fry JC, Hart RJ (1986) An evaluation of the waters Pico-tag system for the amino acid analysis of food materials. J Autom Chem 8:170–177
Yang L, Song Y, Li X, Huang X, Liu J, Ding H, Zhu P, Zhou P (2012) HIV-1 virus-like particles produced by stably transfected Drosophila S2 cells: a desirable vaccine component. J Virol 86:7662–7676. https://doi.org/10.1128/JVI.07164-11
Acknowledgements
Monize Caiado Decarli acknowledges the Coordination for the Improvement of Higher Education Personnel (CAPES/Brazil) for her master fellowship and Daniela Matilde Correia acknowledges CAPES (Atração de Jovens Talentos—Process 064922/2014-01).
Funding
This study was funded by CNPq (National Council for Scientific and Technological Development)—grant number: 402439/2013-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Decarli, M.C., dos Santos, D.P., Astray, R.M. et al. DROSOPHILA S2 cell culture in a WAVE Bioreactor: potential for scaling up the production of the recombinant rabies virus glycoprotein. Appl Microbiol Biotechnol 102, 4773–4783 (2018). https://doi.org/10.1007/s00253-018-8962-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8962-0