Abstract
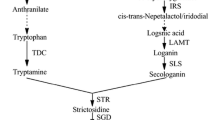
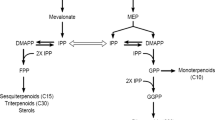
In Catharanthus roseus cell cultures the time courses of four enzyme activities, tryptophan decarboxylase (TDC), strictosidine synthase (SSS), geraniol-10-hydroxylase (G10H) and anthranilate synthase (AS), and alkaloid accumulation were compared under two different culture conditions (low-inoculum density and high-inoculum density on induction medium) and a control on growth medium. In growth medium a transient increase in TDC activity was first observed after which G10H reached its maximum activity; only tryptamine accumulated, no ajmalicine could be detected. Apparently, a concerted induction of enzyme activities is required for ajmalicine formation. Cells inoculated in induction medium showed such a concerted induction of AS, TDC and G10H activities. After 30 days the low-density culture had accumulated six times more ajmalicine (in μmoles/g) than the high-density culture. Thus, increase in biomass concentration (high-density cultures) did not enhance the total alkaloid production. The major differences observed in enzyme levels between high-and low-density cultures were in the AS and TDC activities, which were two to three times higher in the low-density culture, indicating that there is a positive correlation between ajmalicine formation and AS and TDC activities.
Similar content being viewed by others
References
Asada M, Shuler ML (1989) Stimulation of ajmalicine production and excretion from Catharanthus roseus: effects of adsorption in situ, elicitors and alginate immobilization. Appl Microbiol Biotechnol 30:475–481
Berlin J, Mollenschott C, DiCosmo F (1987) Comparison of various strategies designed to optimize indole alkaloid accumulation of a cell suspension culture of Catharanthus roseus. Z Naturforsch 42c:1101–1108
Breuling M, Alfermann AW, Reinhard E (1985) Cultivation of cell cultures of Berberis wilsonae in 20 1 airlift-bioreactors. Plant Cell Rep 4:220–223
Courtois D, Kurkdjian A, Guern J (1980) Tryptamine uptake and accumulation by Catharanthus roseus cells cultivated in liquid medium. Plant Sci Lett 18:85–96
Doireau P, Mérillon JM, Guillot R, Rideau M, Chénieux JC, Brillard M (1987) Time-course studies on indole alkaloid accumulation and changes in tryptophan decarboxylase and strictosidine synthase activities: a comparison in three strains of Catharanthus roseus cells. Planta Med 53:364–367
Drapeau D, Blanch MW, Wilke CR (1987) Economic assessment of plant cell culture for the production of ajmalicine. Biotechnol Bioeng 30:946–953
Facchini JF, DiCosmo F (1991) Secondary metabolite biosynthesis in cultured cells of Catharanthus roseus (L.) G. Don immobilized by adhesion to glass fibers. Appl Microbiol Biotechnol 35:382–392
Goddijn OJM, Kam RF de, Zanetti A, Schilperoort RA, Hoge JHC (1992) Auxin rapidly down-regulates transcription of the tryptophan decarboxylase gene from Catharanthus roseus. Plant Mol Biol 18:1113–1120
Knobloch K-H, Berlin J (1980) Influence of medium composition on the formation of secondary compounds in cell suspension cultures of Catharanthus roseus (L.) G. Don. Z Naturforsch 35c:551–556
Knobloch K-H, Hansen B, Berlin J (1981) Medium-Induced formation of indole alkaloids and concomitant changes of interrelated enzyme activities in cell suspension cultures of Catharanthus roseus. Z Naturforsch 36c:40–43
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Madyastha KM, Meehan TD, Coscia CJ (1976) Characterization of a cytochrome P-450 dependent monoterpene hydroxylase from the higher plant Vinca rosea. Biochemistry 15:1097–1102
Mérillon JM, Doireau P, Guillot A, Chénieux JC, Rideau M (1986) Indole alkaloid accumulation and tryptophan decarboxylase activity in Catharanthus roseus cells cultured in three different media. Plant Cell Rep 5:23–26
Noé W, Berlin J (1985) Induction of de-novo synthesis of tryptophan decarboxylase in cell suspensions of Catharanthus roseus. Planta 166:500–504
Pasquali G, Goddijn OJM, Waal A de, Verpoorte R, Schilperoort RA, Hoge JHC, Memelink J (1992) Coordinated regulation of two indole alkaloid biosynthetic genes from Catharanthus roseus by auxin and elicitors. Plant Mol Biol 18:1121–1131
Pennings EJM, Hegger I, Heijden R van der, Duine J, Verpoorte R (1987) Assay of tryptophan decarboxylase from Catharanthus roseus plant cell cultures by high-performance liquid chromatography. Anal Biochem 165:133–136
Pennings EJM, Bosch RA van den, Heijden R van der, Stevens LH, Duine JA, Verpoorte R (1989) Assay of strictosidine synthase from plant cell cultures by high-performance liquid chromatography. Anal Biochem 176:412–415
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–357
Poulsen C, Pennings EJM, Verpoorte R (1991) High-performance liquid chromatography assay of anthranilate synthase from plant cell cultures. J Chromatogr 547:155–160
Schiel O, Witte L, Berlin J (1987) Geraniol-10-hydroxylase activity and its relation to monoterpene indole alkaloid accumulation in cell suspension cultures of Catharanthus roseus. Z Naturforsch 42c:1075–1081
Schripsema J, Verpoorte R (1992) Search for factors involved in indole alkaloid production in cell suspension cultures of Tabernaemontana divaricata. Planta Med 58:245–249
Schripsema J, Peltenburg-Looman A, Erkelens C, Verpoorte R (1991) Nitrogen metabolism in cultures of Tabernaemontana divaricata. Phytochemistry 30:3951–3954
Stevens LH, Schripsema J, Pennings EJM, Verpoorte R (1992) Activities of enzymes involved in indole alkaloid biosynthesis in suspension cultures of Catharanthus, Cinchona and Tabernaemontana species. Plant Physiol Biochem in press
Tanaka M (1987) Large scale cultivation of plant cells at high density: a review. Process Biochem 8:106–113
Van Gulik WM, Ten Hoopen HJG, Heijnen JJ (1992) Kinetics and stoichiometry of growth of plant cell cultures of Catharanthus roseus and Nicotiana tabacum in batch and continuous fermentors. Biotechnol Bioeng in press
Van der Hrijnen R, Lamping PJ, Out PP, Wijnsma R, Verpoorte R (1987) High-performance liquid chromatographic determination of indole alkaloids in a suspension culture of Tabernaemonta divaricata. J Chromatogr 396:286–295
Zenk MH, El-Shagi H, Arens H, Stöckigt J, Weiler EW, Deus B (1977) Formation of the indole alkaloids serpentine and ajmalicine in cell suspension cultures of Catharanthus roseus. In: Barz W, Reinhard E, Zenk MH (eds) Plant tissue culture and its biotechnological application. Springer, Berlin Heidelberg New York, pp 27–44
Author information
Authors and Affiliations
Additional information
Biotechnology Delft Leiden, Project Group Plant Cell Biotechnology
Correspondence to: R. Verpoorte
Rights and permissions
About this article
Cite this article
Moreno, P.R.H., Schlatmann, J.E., van der Heijden, R. et al. Induction of ajmalicine formation and related enzyme activities in Catharanthus roseus cells: effect of inoculum density. Appl Microbiol Biotechnol 39, 42–47 (1993). https://doi.org/10.1007/BF00166846
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00166846