Abstract
The lateral-flow (immuno)assay (LFA) has been widely investigated for the detection of molecular, macromolecular, and particle targets at the point-of-need due to its ease of use, rapid processing, and minimal power and laboratory equipment requirements. However, for some analytes, such as certain proteins, the detection limit of LFA is inferior to lab-based assays, such as the enzyme-linked immunosorbent assay, and needs to be improved. One solution for improving the detection limit of LFA is to concentrate the target protein in a solution prior to the detection step. In this study, a novel approach was used in the context of an aqueous two-phase micellar system comprised of the nonionic surfactant Triton X-114 to concentrate a model protein, namely transferrin, prior to LFA. Proteins have been shown to partition, or distribute, fairly evenly between the two phases of an aqueous two-phase system, which in turn results in their limited concentration in one of the two phases. Therefore, larger colloidal gold particles decorated with antibodies for transferrin were used in the concentration step to bind to transferrin and aid its partitioning into the top, micelle-poor phase. By manipulating the volume ratio of the two coexisting micellar phases and combining the concentration step with LFA, the transferrin detection limit of LFA was improved by tenfold from 0.5 to 0.05 μg/mL in a predictive manner. In addition to enhancing the sensitivity of LFA, this universal concentration method could also be used to improve other detection assays.

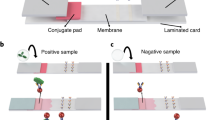
A schematic representation of concentrating a target protein into the top phase of an aqueous two-phase micellar system prior to its detection via the lateral-flow immunoassay






Similar content being viewed by others
References
Shyu RH, Shyu HF, Liu HW, Tang SS (2002) Colloidal gold-based immunochromatographic assay for detection of ricin. Toxicon 40(3):255–258
Chiao DJ, Shyu RH, Hu CS, Chiang HY, Tang SS (2004) Colloidal gold-based immunochromatographic assay for detection of botulinum neurotoxin type B. J Chromatogr 809(1):37–41
Schubert-Ullrich P, Rudolf J, Ansari P, Galler B, Führer M, Molinelli A, Baumgartner S (2009) Commercialized rapid immunoanalytical tests for determination of allergenic food proteins: an overview. Anal Bioanal Chem 395(1):69–81
Peruski AH, Peruski LF Jr (2003) Immunological methods for detection and identification of infectious disease and biological warfare agents. Clin Vaccine Immunol 10(4):506
Albertsson PA (1986) Partition of cell particles and macromolecules, 3rd edn. John Wiley & Sons, New York
Quina FH, Hinze WL (1999) Surfactant-mediated cloud point extractions: an environmentally benign alternative separation approach. Ind Eng Chem Res 38(11):4150–4168
Albertsson PA (1989) Separation of cells and cell organelles by partition in aqueous polymer two-phase systems. Methods Enzymol 171:532–549
Kamei DT, King JA, Wang DI, Blankschtein D (2002) Separating lysozyme from bacteriophage P22 in two-phase aqueous micellar systems. Biotechnol Bioeng 80(2):233–236
Liu CL, Kamei DT, King JA, Wang DI, Blankschtein D (1998) Separation of proteins and viruses using two-phase aqueous micellar systems. J Chromatogr B Biomed Sci Appl 711(1–2):127–138
Ribeiro SC, Monteiro GA, Cabral JM, Prazeres DM (2002) Isolation of plasmid DNA from cell lysates by aqueous two-phase systems. Biotechnol Bioeng 78(4):376–384
Mashayekhi F, Meyer AS, Shiigi SA, Nguyen V, Kamei DT (2009) Concentration of mammalian genomic DNA using two-phase aqueous micellar systems. Biotechnol Bioeng 102(6):1613–1623
Mashayekhi F, Chiu R, Le A, Chao F, Wu B, Kamei D (2010) Enhancing the lateral-flow immunoassay for viral detection using an aqueous two-phase micellar system. Anal Bioanal Chem 398(7):2955–2961
Tanford C (1978) The hydrophobic effect: formation of micelles and biological membranes, 2nd edn. John Wiley & Sons, New York, pp 1–10
Bordier C (1981) Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem 256(4):1604–1607
Nikas YJ, Liu CL, Srivastava T, Abbott NL, Blankschtein D (1992) Protein partitioning in two-phase aqueous nonionic micellar solutions. Macromolecules 25(18):4797–4806
Kamei DT, Liu CL, Haase-Pettingell C, King JA, Wang DI, Blankschtein D (2002) Understanding viral partitioning in two-phase aqueous nonionic micellar systems: 1. Role of attractive interactions between viruses and micelles. Biotechnol Bioeng 78(2):190–202
Kamei DT, Wang DIC, Blankschtein D (2002) Fundamental investigation of protein partitioning in two-phase aqueous mixed (nonionic/ionic) micellar systems. Langmuir 18(8):3047–3057
Albertsson PA, Cajarville A, Brooks DE, Tjerneld F (1987) Partition of proteins in aqueous polymer two-phase systems and the effect of molecular weight of the polymer. Biochim Biophys Acta 926(1):87–93
Frens G (1972) Particle size and sol stability in metal colloids. Colloid Polym Sci 250(7):736–741
Horisberger M, Clerc MF (1985) Labelling of colloidal gold with protein A. A quantitative study. Histochemistry 82(3):219–223
Leuvering JH, Thal PJ, van der Waart M, Schuurs AH (1980) Sol particle immunoassay (SPIA). J Immunoassay 1(1):77–91
Kamei DT, King JA, Wang DI, Blankschtein D (2002) Understanding viral partitioning in two-phase aqueous nonionic micellar systems: 2. Effect of entrained micelle-poor domains. Biotechnol Bioeng 78(2):203–216
Christopher P, Robinson N, Shaw MK (2005) Antibody–label conjugates in lateral-flow assays. In: Forensic science and medicine: drugs of abuse: body fluid testing. Totowa, NJ: Humana Press, 87–98
Jones MN (1992) Surfactant interactions with biomembranes and proteins. Chem Soc Rev 21(2):127–136
Randolph TW, Jones LS (2002) Surfactant–protein interactions rational design of stable protein formulations. In: Carpenter JF, Manning MC (eds) Pharmaceutical Biotechnology, vol 13. Springer, US, pp 159–175. doi:10.1007/978-1-4615-0557-0_7
Albertsson PA (1970) Partition of cell particles and macromolecules in polymer two-phase systems. Adv Protein Chem 24:309–341
Albertsson PA (1958) Partition of proteins in liquid polymer–polymer two-phase systems. Nature 182(4637):709–711
Jyh-Ping C (1992) Partitioning and separation of α-lactalbumin and β-lactoglobulin in PEG/potassium phosphate aqueous two-phase systems. J Ferment Bioeng 73(2):140–147
Acknowledgments
This work was supported by UCLA funds to D.T.K and a Telemedicine and Advanced Technology Research Center (TATRC) research award to F.M.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 137 kb)
Rights and permissions
About this article
Cite this article
Mashayekhi, F., Le, A.M., Nafisi, P.M. et al. Enhancing the lateral-flow immunoassay for detection of proteins using an aqueous two-phase micellar system. Anal Bioanal Chem 404, 2057–2066 (2012). https://doi.org/10.1007/s00216-012-6278-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6278-y