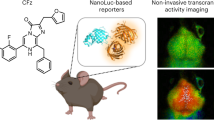
Abstract
The main analytical use of Ca2+-regulated photoproteins from luminous coelenterates is for real-time non-invasive visualization of intracellular calcium concentration ([Ca2+]i) dynamics in cells and whole organisms. A limitation of this approach for in vivo deep tissue imaging is the fact that blue light emitted by the photoprotein is highly absorbed by tissue. Seven novel coelenterazine analogues were synthesized and their effects on the bioluminescent properties of recombinant obelin from Obelia longissima and aequorin from Aequorea victoria were evaluated. Only analogues having electron-donating groups (m-OCH3 and m-OH) on the C6 phenol moiety or an extended resonance system at the C8 position (1-naphthyl and α-styryl analogues) showed a significant red shift of light emission. Of these, only the α-styryl analogue displayed a sufficiently high light intensity to allow eventual tissue penetration. The possible suitability of this compound for in vivo assays was corroborated by studies with aequorin which allowed the monitoring of [Ca2+]i dynamics in cultured CHO cells and in hippocampal brain slices. Thus, the α-styryl coelenterazine analogue might be potentially useful for non-invasive, in vivo bioluminescence imaging in deep tissues of small animals.








Similar content being viewed by others
Abbreviations
- BRET:
-
Bioluminescence Förster resonance energy transfer
- CHO cells:
-
Chinese hamster ovary cells
- CTZ:
-
Coelenterazine
References
Morin JG (1974) In: Muscatine L, Lenhoff HM (eds) Coelenterate biology: reviews and new perspectives. New York, Academic
Vysotski ES, Markova SV, Frank LA (2006) Calcium-regulated photoproteins of marine coelenterates. Mol Biol 40:355–367
Hastings JW, Gibson QH (1963) Intermediates in the bioluminescent oxidation of reduced flavin mononucleotide. J Biol Chem 238:2537–2554
Allen DG, Blinks JR, Prendergast FG (1977) Aequorin luminescence: relation of light emission to calcium concentration – a calcium-independent component. Science 195:996–998
Shimomura O, Johnson FH (1972) Structure of the light-emitting moiety of aequorin. Biochemistry 11:1602–1608
Cormier MJ, Hori K, Karkhanis YD, Anderson JM, Wampler JE, Morin JG, Hastings JW (1973) Evidence for similar biochemical requirements for bioluminescence among the coelenterates. J Cell Physiol 81:291–297
Markova SV, Vysotski ES, Blinks JR, Burakova LP, Wang BC, Lee J (2002) Obelin from the bioluminescent marine hydroid Obelia geniculata: cloning, expression, and comparison of some properties with those of other Ca2+-regulated photoproteins. Biochemistry 41:2227–2236
Head JF, Inouye S, Teranishi K, Shimomura O (2000) The crystal structure of the photoprotein aequorin at 2.3 Å resolution. Nature 405:372–376
Liu ZJ, Vysotski ES, Chen CJ, Rose JP, Lee J, Wang BC (2000) Structure of the Ca2+-regulated photoprotein obelin at 1.7 Å resolution determined directly from its sulfur substructure. Protein Sci 11:2085–2093
Liu ZJ, Vysotski ES, Deng L, Lee J, Rose J, Wang BC (2003) Atomic resolution structure of obelin: soaking with calcium enhances electron density of the second oxygen atom substituted at the C2-position of coelenterazine. Biochem Biophys Res Commun 311:433–439
Titushin MS, Feng Y, Stepanyuk GA, Li Y, Markova SV, Golz S, Wang BC, Lee J, Wang J, Vysotski ES, Liu ZJ (2010) NMR-derived topology of a GFP-photoprotein energy transfer complex. J Biol Chem 285:40891–40900
Deng L, Markova SV, Vysotski ES, Liu ZJ, Lee J, Rose J, Wang BC (2004) Crystal structure of a Ca2+-discharged photoprotein: implications for mechanisms of the calcium trigger and bioluminescence. J Biol Chem 279:33647–33652
Deng L, Vysotski ES, Markova SV, Liu ZJ, Lee J, Rose J, Wang BC (2005) All three Ca2+-binding loops of photoproteins bind calcium ions: the crystal structures of calcium-loaded apo-aequorin and apo-obelin. Protein Sci 14:663–675
Liu ZJ, Stepanyuk GA, Vysotski ES, Lee J, Markova SV, Malikova NP, Wang BC (2006) Crystal structure of obelin after Ca2+-triggered bioluminescence suggests neutral coelenteramide as the primary excited state. Proc Natl Acad Sci U S A 103:2570–2575
Vysotski ES, Liu ZJ, Markova SV, Blinks JR, Deng L, Frank LA, Herko M, Malikova NP, Rose JP, Wang BC, Lee J (2003) Violet bioluminescence and fast kinetics from W92F obelin: structure-based proposals for the bioluminescence triggering and the identification of the emitting species. Biochemistry 42:6013–6024
Vysotski ES, Lee J (2004) Ca2+-regulated photoproteins: structural insight into the bioluminescence mechanism. Acc Chem Res 37:405–415
Vysotski ES, Lee J (2007) In: Viviani VR, Ohmiya Y (eds) Luciferases and fluorescent proteins: principles and advances in biotechnology and bioimaging. Transworld Research Network, Kerala
Blinks JR, Wier WG, Hess P, Prendergast FG (1982) Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol 40:1–114
Pinton P, Rimessi A, Romagnoli A, Prandini A, Rizzuto R (2007) Biosensors for the detection of calcium and pH. Methods Cell Biol 80:297–325
Eglen RM, Reisine T (2008) Photoproteins: important new tools in drug discovery. Assay Drug Dev Technol 6:659–671
Ottolini D, Calì T, Brini M (2013) Measurements of Ca2+ concentration with recombinant targeted luminescent probes. Methods Mol Biol 937:273–91
Webb SE, Rogers KL, Karplus E, Miller AL (2010) The use of aequorins to record and visualize Ca2+ dynamics: from subcellular microdomains to whole organisms. Methods Cell Biol 99:263–300
Weissleder R, Ntziachristos V (2003) Shedding light onto live molecular targets. Nat Med 9:123–128
Baubet V, Le Mouellic H, Campbell AK, Lucas-Meunier E, Fossier P, Brûlet P (2000) Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+reporters at the single-cell level. Proc Natl Acad Sci U S A 97:7260–7265
Curie T, Rogers KL, Colasante C, Brûlet P (2007) Red-shifted aequorin-based bioluminescent reporters for in vivo imaging of Ca2+ signaling. Mol Imaging 6:30–42
Bakayan A, Vaquero CF, Picazo F, Llopis J (2011) Red fluorescent protein-aequorin fusions as improved bioluminescent Ca2+ reporters in single cells and mice. PLoS One 6:e19520
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, Singapore
Rogers KL, Picaud S, Roncali E, Boisgard R, Colasante C, Stinnakre J, Tavitian B, Brûlet P (2007) Non-invasive in vivo imaging of calcium signaling in mice. PLoS One 3:e974
Martin JR, Rogers KL, Chagneau C, Brûlet P (2007) In vivo bioluminescence imaging of Ca2+signalling in the brain of Drosophila. PLoS One 2:e275
Naumann EA, Kampff AR, Prober DA, Schier AF, Engert F (2010) Monitoring neural activity with bioluminescence during natural behavior. Nat Neurosci 23:513–520
Shimomura O (2006) Bioluminescence: chemical principles and methods. World Scientific, Singapore
Inouye S, Shimomura O (1997) The use of Renilla luciferase, Oplophorus luciferase, and apoaequorin as bioluminescent reporter protein in the presence of coelenterazine analogues as substrate. Biochem Biophys Res Commun 233:349–353
Loening AM, Wu AM, Gambhir SS (2007) Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat Methods 4:641–643
Stepanyuk GA, Unch J, Malikova NP, Markova SV, Lee J, Vysotski ES (2010) Coelenterazine-v ligated to Ca2+-triggered coelenterazine-binding protein is a stable and efficient substrate of the red-shifted mutant of Renilla muelleri luciferase. Anal Bioanal Chem 398:1809–1817
Illarionov BA, Markova SV, Bondar VS, Vysotski ES, Gitelson JI (1992) Isolation and expression of cDNA coding for photoprotein obelin from hydroid Obelia longissima. Dokl Akad Nauk 326:911–913
Illarionov BA, Bondar VS, Illarionova VA, Vysotski ES (1995) Sequence of the cDNA encoding the Ca2+-activated photoprotein obelin from the hydroid polyp Obelia longissima. Gene 153:273–274
Prasher D, McCann RO, Cormier MJ (1985) Cloning and expression of the cDNA coding for aequorin, a bioluminescent calcium-binding protein. Biochem Biophys Res Commun 126:1259–1268
Adamczyk M, Johnson DD, Mattingly PG, Pan Y, Reddy RE (2001) Synthesis of coelenterazine. Org Prep Proc Int 33:477–485
Adamczyk M, Akireddy SR, Johnson DD, Mattingly PG, Pan Y, Reddy RE (2003) Synthesis of 3,7-dihydroimidazo[1,2a]pyrazine-3-ones and their chemiluminescent properties. Tetrahedron 59:8129–8142
Tao Z-F, Li G, Tong Y, Chen Z, Merta P, Kovar P, Zhang H, Rosenberg SH, Sham HL, Sowin TJ, Lin N-H (2007) Synthesis and biological evaluation of 4'-(6,7-disubstituted-2,4-dihydro-indeno[1,2-c]pyrazol-3-yl)-biphenyl-4-ol as potent Chk1 inhibitors. Bioorg Med Chem Lett 17:4308–4315
Hirano T, Ohmiya Y, Maki S, Niwa H, Ohashi M (1998) Bioluminescent properties of fluorinated semi-synthetic Aequorins. Tetrahedron Lett 39:5541–5544
Illarionov BA, Frank LA, Illarionova VA, Bondar VS, Vysotski ES, Blinks JR (2000) Recombinant obelin: cloning and expression of cDNA purification, and characterization as a calcium indicator. Methods Enzymol 305:223–249
Vysotski ES, Liu ZJ, Rose J, Wang BC, Lee J (2001) Preparation and X-ray crystallographic analysis of recombinant obelin crystals diffracting to beyond 1.1 Å. Acta Crystallogr D Biol Crystallogr 57:1919–1921
Eremeeva EV, Markova SV, Frank LA, Visser AJ, van Berkel WJ, Vysotski ES (2013) Bioluminescent and spectroscopic properties of His-Trp-Tyr triad mutants of obelin and aequorin. Photochem Photobiol Sci 12:1016–1024
Klabusay M, Blinks JR (1996) Some commonly overlooked properties of calcium buffer systems: a simple method for detecting and correcting stoichiometric imbalance in CaEGTA stock solutions. Cell Calcium 20:227–234
Rogers KL, Stinnakre J, Agulhon C, Jublot D, Shorte SL, Kremer EJ, Brûlet P (2005) Visualization of local Ca2+ dynamics with genetically encoded bioluminescent reporters. Eur J Neurosci 21(3):597–610
Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schütz G (2002) Disruption of CREB function in brain leads to neurodegeneration. Nat Genet 31:47–54
Shimomura O, Johnson FH (1970) Calcium binding, quantum yield, and emitting molecule in aequorin bioluminescence. Nature 227:1356–1357
Shimomura O, Musicki B, Kishi Y, Inouye S (1993) Light-emitting properties of recombinant semi-synthetic aequorins and recombinant fluorescein conjugated aequorin for measuring cellular calcium. Cell Calcium 14:373–378
Stepanyuk GA, Golz S, Markova SV, Frank LA, Lee J, Vysotski ES (2005) Interchange of aequorin and obelin bioluminescence color is determined by substitution of one active site residue of each photoprotein. FEBS Lett 579:1008–1014
Shimomura O (1997) Membrane permeability of coelenterazine analogues measured with fish eggs. Biochem J 326:297–298
Bovolenta S, Foti M, Lohmer S, Corazza S (2007) Development of a Ca2+-activated photoprotein, Photina, and its application to high-throughput screening. J Biomol Screen 12:694–704
Sabatini BL, Oertner TG, Svoboda K (2002) The life cycle of Ca2+ ions in dendritic spines. Neuron 33:439–452
Shimomura O (1991) Preparation and handling of aequorin solutions for the measurement of cellular Ca2+. Cell Calcium 12:635–643
Ohmiya Y, Ohashi M, Tsuji FI (1992) Two excited states in aequorin bioluminescence induced by tryptophan modification. FEBS Lett 301:197–201
Giuliani G, Molinari P, Ferretti G, Cappelli A, Anzini M, Vomero S, Costa T (2012) New red-shifted coelenterazine analogues with an extended electronic conjugation. Tetrahedron Lett 53:5114–5118
Shimomura O, Musicki B, Kishi Y (1989) Semi-synthetic aequorins with improved sensitivity to Ca2+ ions. Biochem J 261:913–920
Acknowledgements
R.G. acknowledges the ICSN for a fellowship. We are grateful for the ANR grant to P.B. and a CNRS Physics, Chemistry and Biology interface grant to R.H.D. and P.B.; N.P.M, L.P.B., and E.S.V. acknowledge the RFBR grant 12-04-00131 and the Program of the Government of Russian Federation “Measures to attract leading scientists to Russian educational institutions” (grant 11.G34.31.0058). P.B. and A.J.B. are indebted to Eric Karplus from Science Wares Inc. for helping with single-photon imaging software.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ronan Gealageas, Natalia P. Malikova, and Sandrine Picaud contributed equally to this work.
Philippe Brûlet is deceased.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 266 kb)
Rights and permissions
About this article
Cite this article
Gealageas, R., Malikova, N.P., Picaud, S. et al. Bioluminescent properties of obelin and aequorin with novel coelenterazine analogues. Anal Bioanal Chem 406, 2695–2707 (2014). https://doi.org/10.1007/s00216-014-7656-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7656-4