Feasibility Study on the Use of Recycled Polymers for Malathion Adsorption: Isotherms and Kinetic Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbate
2.2. Preparation of Adsorbent Materials
2.2.1. Activated Carbon
2.2.2. Polyvinylchloride
2.2.3. Polystyrene
2.3. Experimental Design
2.4. Quantification of the Adsorbate
2.5. Adsorption Kinetics
2.6. Equilibrium Studies (Isotherms)
2.7. SEM/EDX Analysis
2.8. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.9. Thermogravimetric and Differential Thermal Analysis (TG/DTA)
3. Results and Discussion
3.1. Statistical Analysis
3.2. Adsorption Kinetics
3.3. Adsorption Isotherms
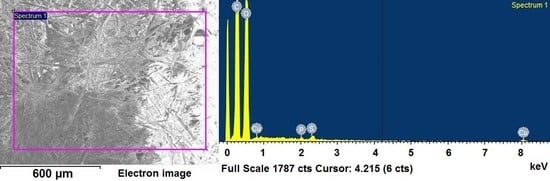
3.4. SEM/EDX Analysis
3.5. Fourier Transform Infrared Spectroscopy (FTIR)
3.6. Thermogravimetric and Differential Thermal Analysis (TG/DTA)
3.7. Forthcoming Investigations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ioannidou, O.A.; Zabaniotou, A.A.; Stavropoulos, G.G.; Islam, M.A.; Albanis, T.A. Preparation of activated carbons from agricultural residues for pesticide adsorption. Chemosphere 2010, 80, 1328–1336. [Google Scholar] [CrossRef]
- Vukčević, M.M.; Kalijadis, A.M.; Vasiljević, T.M.; Babić, B.M.; Laušević, Z.V.; Laušević, M.D. Production of activated carbon derived from waste hemp (Cannabis sativa) fibers and its performance in pesticide adsorption. Microporous Mesoporous Mat. 2015, 214, 156–165. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. New models for kinetics and equilibrium homogeneous adsorption. Chem. Eng. Res. Des. 2016, 112, 289–297. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Diez, M.; Gryglewicz, G. Influence of pore size distribution on the adsorption of phenol on PET-based activated carbons. J. Colloid Interface Sci. 2016, 469, 205–212. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Yousif, A.M. Adsorption of malathion on thermally treated egg shell material. Water. Sci. Technol. 2010, 61, 1035–1041. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Krishna, S.K.; Kalaamanic, P.; Subburamaan, C.V.; Subramaniam, N.G.; Kang, T.W. Kinetic Approach for the Adsorption of Organophosphorous Pesticides from Aqueous Solution Using “Waste” Jute Fiber Carbon. E- J. Chem 2010, 7 (Suppl. 1), S511–S519. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A. Modification and characterization of PET fibers for fast removal of Hg (II), Cu (II) and Co (II) metal ions from aqueous solutions. J. Hazard. Mater. 2013, 250, 122–130. [Google Scholar] [CrossRef]
- Al-Jabari, M. Kinetic models for adsorption on mineral particles comparison between Langmuir kinetics and mass transfer. Environ. Technol. Innov. 2016, 6, 27–37. [Google Scholar] [CrossRef]
- Cansado, I.P.; Galacho, C.; Nunes, Â.S.; Carrott, M.L.; Carrott, P.J. Adsorption properties of activated carbons prepared from recycled PET in the removal of organic pollutants from aqueous solutions. Adsorpt. Sci. Technol. 2010, 28, 807–821. [Google Scholar] [CrossRef]
- Mendoza-Carrasco, R.; Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C.; Gómez-Serrano, V. Preparation of high-quality activated carbon from polyethyleneterephthalate (PET) bottle waste. Its use in the removal of pollutants in aqueous solution. J. Environ. Manag. 2016, 181, 522–535. [Google Scholar] [CrossRef]
- Ji, L.; Liu, F.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of pharmaceutical antibiotics on template-synthesized ordered micro-and mesoporous carbons. Environ. Sci. Technol. 2010, 44, 3116–3122. [Google Scholar] [CrossRef]
- Demirbas, E.; Dizge, N.; Sulak, M.T.; Kobya, M. Adsorption kinetics and equilibrium of copper from aqueous solutions using hazelnut shell activated carbon. Chem. Eng. J. 2009, 148, 480–487. [Google Scholar] [CrossRef]
- Benjwal, P.; Sharma, R.; Kar, K.K. Effects of surface microstructure and chemical state of featherfiber-derived multidoped carbon fibers on the adsorption of organic water pollutants. Mater. Des. 2016, 110, 762–774. [Google Scholar] [CrossRef]
- Taha, S.M.; Amer, M.E.; Elmarsafy, A.E.; Elkady, M.Y. Adsorption of 15 different pesticides on untreated and phosphoric acid treated biochar and charcoal from water. J. Environ. Chem. Eng. 2014, 2, 2013–2025. [Google Scholar] [CrossRef]
- Leyva Morales, J.B.; Garcia de la Parra, L.M.; Bastidas Bastidas, P.D.J.; Astorga Rodríguez, J.E.; Bejarano Trujillo, J.; Cruz Hernández, A.; Rodríguez, I.E.M.; Betancourt Lozano, M. Uso de plaguicidas en un valle agrícola tecnificado en el noroeste de México. Rev. Int. Contam. Ambient. 2014, 30, 247–261. [Google Scholar]
- Hernández-Antonio, A.; Hansen, A.M. Uso de plaguicidas en dos zonas agrícolas de México y evaluación de la contaminación de agua y sedimentos. Rev. Int. Contam. Ambient. 2011, 27, 115–127. [Google Scholar]
- Jusoh, A.; Hartini, W.J.H.; Endut, A. Study on the removal of pesticide in agricultural run off by granular activated carbon. Bioresour. Technol 2011, 102, 5312–5318. [Google Scholar] [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; Al-Tamrah, S.A.; Ghafar, A.A.; Soylak, M. Activated carbon from waste as an efficient adsorbent for malathion for detection and removal purposes. J. Ind. Eng. Chem. 2015, 32, 336–344. [Google Scholar] [CrossRef]
- Mirković, M.M.; Pašti, T.L.; Došen, A.M.; Čebela, M.Ž.; Rosić, A.A.; Matović, B.Z.; Babić, B.M. Adsorption of malathion on mesoporous monetite obtained by mechanochemical treatment of brushite. RSC Adv. 2016, 6, 12219–12225. [Google Scholar] [CrossRef]
- Harper, W.F., Jr.; Flemings, W.; Bailey, K.; Lee, W.; Felker, D.; Gallardo, V.; Magnuson, M.; Phillips, R. Adsorption of Malathion Onto Copper and Iron Surfaces Relevant to Water Infrastructure. J. Am. Water Works Assoc. 2017, 109, E494–E502. [Google Scholar] [CrossRef]
- Wanjeri, V.W.O.; Sheppard, C.J.; Prinsloo, A.R.E.; Ngila, J.C.; Ndungu, P.G. Isotherm and kinetic investigations on the adsorption of organophosphorus pesticides on graphene oxide based silica coated magnetic nanoparticles functionalized with 2-phenylethylamine. J. Environ. Chem. Eng. 2018, 6, 1333–1346. [Google Scholar] [CrossRef]
- Hameed, B.; Salman, J.; Ahmad, A. Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones. J. Hazard. Mater. 2009, 163, 121–126. [Google Scholar] [CrossRef]
- Bolboaca, S.D.; Jäntschi, L. Design of experiments: Useful orthogonal arrays for number of experiments from 4 to 16. Entropy 2007, 9, 198–232. [Google Scholar] [CrossRef] [Green Version]
- Naushad, M.; ALOthman, Z.; Khan, M.; ALQahtani, N.; ALSohaimi, I. Equilibrium, kinetics and thermodynamic studies for the removal of organophosphorus pesticide using Amberlyst-15 resin: Quantitative analysis by liquid chromatography–mass spectrometry. J. Ind. Eng. Chem. 2014, 20, 4393–4400. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Yagub, M.; Kanti, S.T.; Afroze, S.; Ang, H. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, H.; Kapur, M.; Mondal, M.K. Comparative study of malathion removal from aqueous solution by agricultural and commercial adsorbents. J. Water Process Eng. 2014, 3, 67–73. [Google Scholar] [CrossRef]
- Rivas, C.; Núñez, O.; Longoria, F.; Gonzalez, L. Langmuir and freundlich isotherms as model for the adsorption of nucleic acid components on WO3. Saber 2014, 26, 43–49. [Google Scholar]
- Algothmi, W.; Murthy Bandaru, N.; Yu, Y.; Shapter, J.; Ellis, A. Alginate-graphene oxide hybrid gel beads: An efficient copper adsorbent material. J. Colloid Interface Sci. 2013, 397, 32–38. [Google Scholar] [CrossRef]
- Fu, G.-D.; Li, G.L.; Neoh, K.G.; Kang, E.T. Hollow polymeric nanostructures—Synthesis, morphology and function. Prog. Polym. Sci. 2011, 36, 127–167. [Google Scholar] [CrossRef]
- Bhagya Sree, K.; Madhava Kumar, Y.; Gopal, N.; Ramu, C. Preparation and characterization of pure and copper-doped PVC films. J. Polym. Eng. 2017, 37, 83–92. [Google Scholar] [CrossRef]





| Volume Occupied by the Dry Adsorbent Materials (cm3) | Mass of Dry Activated Carbon (g) | Mass of Dry PVC (g) | Mass of Dry HDPS (g) |
|---|---|---|---|
| 16.95 | 7.50 | 0.55 | 0.23 |
| 15.07 | 6.66 | 0.50 | 0.20 |
| 13.18 | 5.83 | 0.45 | 0.18 |
| 11.30 | 5.00 | 0.40 | 0.16 |
| 9.42 | 4.16 | 0.35 | 0.13 |
| 7.53 | 3.33 | 0.30 | 0.11 |
| 5.65 | 2.50 | 0.25 | 0.09 |
| Treatment | Adsorbent Material | Temperature (°C) | Volume of the Dry Adsorbent Material (cm3) | Concentration Removed (mg/L) | Efficiency |
|---|---|---|---|---|---|
| Factor A | Factor B | Factor C | |||
| 1 | AC | 30 | 11.30 | 14.74 | 38% |
| 2 | PVC | 30 | 5.65 | 16.08 | 40% |
| 3 | HDPS | 40 | 5.65 | 13.06 | 33% |
| 4 | AC | 20 | 5.65 | 6.17 | 18% |
| 5 | PVC | 20 | 16.95 | 40.48 | 96% |
| 6 | PVC | 40 | 11.30 | 32.92 | 83% |
| 7 | AC | 40 | 16.95 | 38.74 | 96% |
| 8 | HDPS | 30 | 16.95 | 38.87 | 96% |
| 9 | HDPS | 20 | 11.30 | 36.50 | 90% |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | p-Value |
|---|---|---|---|---|
| Factor | 214.0261 | 1 | 214.0261 | 0.0308 |
| Factor | 982.8551 | 1 | 982.8551 | 0.0021 |
| Error | 80.1261 | 4 | 20.0315 | |
| Total | 1364.525 | 8 |
| Coefficient | Units | Kumar | Habila | This Study |
|---|---|---|---|---|
| et al. [27] | et al. [18] | (PVC) | ||
| Dimensionless | 0.996 | 0.98 | 0.959 | |
| mg/g | 21.74 | 32.11 | 96.15 | |
| b | L/mg | 0.53 | — | 0.001 |
| Dimensionless | 0.54 | — | 0.944 |
| Element | Before Adsorption Process % Weight | Before Adsorption Process % Atomic | After Adsorption Process % Weight | After Adsorption Process % Atomic |
|---|---|---|---|---|
| C | 45.6 | 52.76 | 47.32 | 54.56 |
| O | 54.36 | 47.22 | 52.41 | 45.37 |
| Al | 0.04 | 0.02 | 0 | 0 |
| P | 0 | 0 | 0.03 | 0.01 |
| S | 0 | 0 | 0.05 | 0.02 |
| Cu | 0 | 0 | 0.19 | 0.04 |
| Total | 100 | 100 | 100 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermosillo-Nevárez, J.J.; Bustos-Terrones, V.; Bustos-Terrones, Y.A.; Uriarte-Aceves, P.M.; Rangel-Peraza, J.G. Feasibility Study on the Use of Recycled Polymers for Malathion Adsorption: Isotherms and Kinetic Modeling. Materials 2020, 13, 1824. https://doi.org/10.3390/ma13081824
Hermosillo-Nevárez JJ, Bustos-Terrones V, Bustos-Terrones YA, Uriarte-Aceves PM, Rangel-Peraza JG. Feasibility Study on the Use of Recycled Polymers for Malathion Adsorption: Isotherms and Kinetic Modeling. Materials. 2020; 13(8):1824. https://doi.org/10.3390/ma13081824
Chicago/Turabian StyleHermosillo-Nevárez, Jhonatan J., Victoria Bustos-Terrones, Yaneth A. Bustos-Terrones, Perla Marysol Uriarte-Aceves, and Jesus Gabriel Rangel-Peraza. 2020. "Feasibility Study on the Use of Recycled Polymers for Malathion Adsorption: Isotherms and Kinetic Modeling" Materials 13, no. 8: 1824. https://doi.org/10.3390/ma13081824