Enhancing Stability of Microalgae Biocathode by a Partially Submerged Carbon Cloth Electrode for Bioenergy Production from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism, Medium, and Wastewater
2.2. Configuration of Microbial Fuel Cells
2.3. Operation of Microbial Fuel Cells
2.4. Analytical Methods
3. Results and Discussion
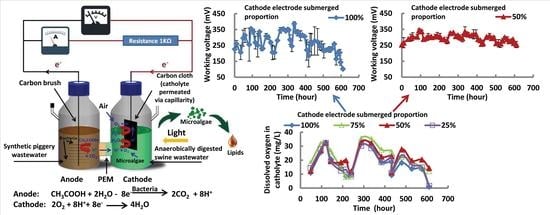
3.1. Effect of the Proportion of Cathode Electrode Submerged on Working Voltage
3.2. Effect of the Proportion of Cathode Electrode Submerged on Electrical Power Density and Internal Resistance
3.3. Biomass and Lipid Production of Microalgae in Biocathode with Partially and Completely Submerged Cathode Electrodes
3.4. Influence of the Proportion of Electrode Submerged on Improvement in COD to Nitrogen Ratio for Wastewater in Biocathode
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|
| Between Groups | 0.139 | 3 | 0.046 | 23.927 | 0.000 |
| Within Groups | 0.796 | 412 | 0.002 | N/A | N/A |
| Total | 0.935 | 415 | N/A | N/A | N/A |
| (I) Sub_Prop | (J) Sub_Prop | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 25 | 50 | −0.0183048 * | 0.0059198 | 0.002 | −0.029943 | −0.006667 |
| 75 | 0.0361045 * | 0.0061457 | 0.000 | 0.024022 | 0.048187 | |
| 100 | 0.0107087 | 0.0059198 | 0.071 | −0.000929 | 0.022347 | |
| 50 | 25 | 0.0183048 * | 0.0059198 | 0.002 | 0.006667 | 0.029943 |
| 75 | 0.0544093 * | 0.0061457 | 0.000 | 0.042327 | 0.066491 | |
| 100 | 0.0290135 * | 0.0059198 | 0.000 | 0.017375 | 0.040652 | |
| 75 | 25 | −0.0361045 * | 0.0061457 | 0.000 | −0.048187 | −0.024022 |
| 50 | −0.0544093 * | 0.0061457 | 0.000 | −0.066491 | −0.042327 | |
| 100 | −0.0253958 * | 0.0061457 | 0.000 | −0.037478 | −0.013314 | |
| 100 | 25 | −0.0107087 | 0.0059198 | 0.071 | −0.022347 | 0.000929 |
| 50 | −0.0290135 * | 0.0059198 | 0.000 | −0.040652 | −0.017375 | |
| 75 | 0.0253958 * | 0.0061457 | 0.000 | 0.013314 | 0.037478 | |
| Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|
| Between Groups | 1032.375 | 3 | 344.125 | 1.066 | 0.457 |
| Within Groups | 1291.500 | 4 | 322.875 | N/A | N/A |
| Total | 2323.875 | 7 | N/A | N/A | N/A |
| (I) Sub_Prop | (J) Sub_Prop | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 25 | 50 | −17.000 | 17.969 | 0.398 | −66.89 | 32.89 |
| 75 | 15.000 | 17.969 | 0.451 | −34.89 | 64.89 | |
| 100 | 1.500 | 17.969 | 0.937 | −48.39 | 51.39 | |
| 50 | 25 | 17.000 | 17.969 | 0.398 | −32.89 | 66.89 |
| 75 | 32.000 | 17.969 | 0.150 | −17.89 | 81.89 | |
| 100 | 18.500 | 17.969 | 0.361 | −31.39 | 68.39 | |
| 75 | 25 | −15.000 | 17.969 | 0.451 | −64.89 | 34.89 |
| 50 | −32.000 | 17.969 | 0.150 | −81.89 | 17.89 | |
| 100 | −13.500 | 17.969 | 0.494 | −63.39 | 36.39 | |
| 100 | 25 | −1.500 | 17.969 | 0.937 | −51.39 | 48.39 |
| 50 | −18.500 | 17.969 | 0.361 | −68.39 | 31.39 | |
| 75 | 13.500 | 17.969 | 0.494 | −36.39 | 63.39 | |
| Time (hour) | 48 | 96 | 120 | 144 | 192 | 216 | 240 | 288 | 336 | 384 | 432 | 432 | 480 | 528 | 576 | 610 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Submerged Proportion (%) | |||||||||||||||||
| 25 | 0.7 | 5.4 | 0.4 | 1.8 | 4.7 | 0.3 | 0.9 | 0.6 | 4.1 | 1.3 | 0.3 | 0.2 | 6.1 | 2.0 | 2.6 | 1.6 | |
| 50 | 0.3 | 6.5 | 0.5 | 3.1 | 8.3 | 4.6 | 2.3 | 0.2 | 1.5 | 2.8 | 2.3 | 1.2 | 0.8 | 2.0 | 5.1 | 2.3 | |
| 75 | 0.7 | 4.0 | 0.4 | 1.7 | 4.7 | 2.0 | 1.5 | 1.3 | 0.3 | 1.9 | 0.0 | 1.4 | 0.6 | 0.6 | 6.8 | 1.9 | |
| 100 | 1.2 | 0.1 | 0.5 | 0.3 | 1.1 | 1.2 | 1.2 | 0.4 | 1.2 | 2.6 | 5.6 | 2.8 | 2.5 | 6.1 | 6.2 | 1.0 | |


References
- Li, S.; Chen, G.; Anandhi, A. Applications of emerging bioelectrochemical technologies in agricultural systems: A current review. Energies 2018, 11, 2951. [Google Scholar] [CrossRef]
- Ali, J.; Sohail, A.; Wang, L.; Rizwan Haider, M.; Mulk, S.; Pan, G. Electro-microbiology as a promising approach towards renewable energy and environmental sustainability. Energies 2018, 11, 1822. [Google Scholar] [CrossRef]
- López Zavala, M.; Torres Delenne, P.; González Peña, O. Improvement of wastewater treatment performance and power generation in microbial fuel cells by enhancing hydrolysis and acidogenesis, and by reducing internal losses. Energies 2018, 11, 2309. [Google Scholar] [CrossRef]
- Włodarczyk, P.; Włodarczyk, B. Microbial fuel cell with ni–co cathode powered with yeast wastewater. Energies 2018, 11, 3194. [Google Scholar] [CrossRef]
- Onumaegbu, C.; Alaswad, A.; Rodriguez, C.; Olabi, A. Optimization of pre-treatment process parameters to generate biodiesel from microalga. Energies 2018, 11, 806. [Google Scholar] [CrossRef]
- Shukla, M.; Kumar, S. Algal growth in photosynthetic algal microbial fuel cell and its subsequent utilization for biofuels. Renew. Sustain. Energy Rev. 2018, 82, 402–414. [Google Scholar] [CrossRef]
- Chandra, R.; Sravan, J.S.; Hemalatha, M.; Kishore Butti, S.; Venkata Mohan, S. Photosynthetic synergism for sustained power production with microalgae and photobacteria in a biophotovoltaic cell. Energy Fuels 2017, 31, 7635–7644. [Google Scholar] [CrossRef]
- Commault, A.S.; Laczka, O.; Siboni, N.; Tamburic, B.; Crosswell, J.R.; Seymour, J.R.; Ralph, P.J. Electricity and biomass production in a bacteria-chlorella based microbial fuel cell treating wastewater. J. Power Sources 2017, 356, 299–309. [Google Scholar] [CrossRef]
- Hou, Q.; Pei, H.; Hu, W.; Jiang, L.; Yu, Z. Mutual facilitations of food waste treatment, microbial fuel cell bioelectricity generation and chlorella vulgaris lipid production. Bioresour. Technol. 2016, 203, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.F.; Lin, J.Y.; Pan, K.Y.; Huang, C.K.; Chu, Y.K. Overexpressing ferredoxins in chlamydomonas reinhardtii increase starch and oil yields and enhance electric power production in a photo microbial fuel cell. Int. J. Mol. Sci. 2015, 16, 19308–19325. [Google Scholar] [CrossRef]
- Kakarla, R.; Min, B. Evaluation of microbial fuel cell operation using algae as an oxygen supplier: Carbon paper cathode vs. Carbon brush cathode. Bioprocess Biosyst. Eng. 2014, 37, 2453–2461. [Google Scholar] [CrossRef] [PubMed]
- Bazdar, E.; Roshandel, R.; Yaghmaei, S.; Mardanpour, M.M. The effect of different light intensities and light/dark regimes on the performance of photosynthetic microalgae microbial fuel cell. Bioresour. Technol. 2018, 261, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Rago, L.; Cristiani, P.; Villa, F.; Zecchin, S.; Colombo, A.; Cavalca, L.; Schievano, A. Influences of dissolved oxygen concentration on biocathodic microbial communities in microbial fuel cells. Bioelectrochemistry 2017, 116, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, H.; Zhao, C.; Huang, F.; Deng, L.; Wang, W. Establishment of stable microalgal-bacterial consortium in liquid digestate for nutrient removal and biomass accumulation. Bioresour. Technol. 2018, 268, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-L.; Chen, Y.-P.; Yu, M.-C.; Hsieh, S.-Y.; Hwang, I.-E.; Liu, Y.-J.; Huang, C.-Y.; Tseng, C.-P.; Liaw, L.-L. Biomass and lipid production of a novel microalga, chlorellaceae sp. P5, through heterotrophic and swine wastewater cultivation. J. Renew. Sustain. Energy 2018, 10, 33102. [Google Scholar] [CrossRef]
- Sotres, A.; Tey, L.; Bonmati, A.; Vinas, M. Microbial community dynamics in continuous microbial fuel cells fed with synthetic wastewater and pig slurry. Bioelectrochemistry 2016, 111, 70–82. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Shun, N.Y.Z. Analysis of Fatty Acids in Infant Formulas Using an Agilent J & W HP-88 Capillary Gc Column. Available online: https://www.agilent.com/cs/library/applications/5990-8429EN.pdf (accessed on 31 October 2017).
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Administration: Washington, DC, USA, 2005. [Google Scholar]
- Maity, J.P.; Hou, C.-P.; Majumder, D.; Bundschuh, J.; Kulp, T.R.; Chen, C.-Y.; Chuang, L.-T.; Nathan Chen, C.-N.; Jean, J.-S.; Yang, T.-C.; et al. The production of biofuel and bioelectricity associated with wastewater treatment by green algae. Energy 2014, 78, 94–103. [Google Scholar] [CrossRef]
- Amini, H.; Wang, L.J.; Shahbazi, A. Effects of harvesting cell density, medium depth and environmental factors on biomass and lipid productivities of chlorella vulgaris grown in swine wastewater. Chem. Eng. Sci. 2016, 152, 403–412. [Google Scholar] [CrossRef]
- Olguin, E.J.; Castillo, O.S.; Mendoza, A.; Tapia, K.; Gonzalez-Portela, R.E.; Hernandez-Landa, V.J. Dual purpose system that treats anaerobic effluents from pig waste and produce neochloris oleoabundans as lipid rich biomass. New Biotechnol. 2015, 32, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Fattah, I.M.R.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Carrera, J.; Vicent, T.; Lafuente, J. Effect of influent cod/n ratio on biological nitrogen removal (bnr) from high-strength ammonium industrial wastewater. Process Biochem. 2004, 39, 2035–2041. [Google Scholar] [CrossRef]



| Submerged Proportion of Cathode Electrode | 25% | 50% | 75% | 100% | |
|---|---|---|---|---|---|
| Working voltage on 1 kΩ (mV) | max | 329 | 344 | 322 | 387 |
| average | 278 ± 32 | 297 ± 26 | 246 ± 41 | 268 ± 66 | |
| Max power density (mW/m3) | max | 84 | 103 | 76 | 103 |
| average | 82 ± 4 | 99 ± 6 | 67 ± 13 | 80 ± 33 | |
| Current density (mA/m3) | max | 614 | 512 | 506 | 577 |
| average | 410 ± 51 | 446 ± 34 | 375 ± 47 | 410 ± 96 | |
| Internal resistance (× 102 Ω) | average | 7 ± 1 | 6 ± 0.1 | 7 ± 1 | 7 ± 2 |
| Biomass production (dry weight, mg/L) | max | 628 ± 33 | 616 ± 17 | 647 ± 57 | 659 ± 12 |
| average | 573 ± 53 | 556 ± 74 | 572 ± 103 | 587 ± 83 | |
| Lipid production (mg/L) | max | 214 ± 4 | 250 ± 42 | 232 ± 2 | 222 ± 15 |
| average | 206 ± 8 | 209 ± 37 | 205 ± 30 | 199 ± 27 | |
| Lipid content (%) | max | 40 ± 3 | 41 ± 6 | 38 ± 4 | 37 ± 2 |
| average | 36 ± 3 | 38 ± 3 | 36 ± 2 | 34 ± 3 | |
| NH3-N Removal (%) | max | 62 ± 1 | 60 ± 1 | 58 ± 1 | 63 ± 2 |
| average | 54 ± 28 | 55 ± 8 | 52 ± 6 | 56 ± 7 | |
| TN Removal (%) | max | 62 ± 1 | 75 ± 1 | 68 ± 3 | 68 ± 7 |
| average | 52 ± 10 | 62 ± 11 | 59 ± 8 | 59 ± 10 | |
| COD/TN ratio of effluent | average | 2.0 ± 0.3 | 2.3 ± 0.5 | 2.4 ± 0.6 | 2.3 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, J.; Xu, Y.; Lu, C.; Lai, W.; Xie, G.; Zheng, L.; Talawar, M.P.; Du, Q.; Li, G. Enhancing Stability of Microalgae Biocathode by a Partially Submerged Carbon Cloth Electrode for Bioenergy Production from Wastewater. Energies 2019, 12, 3229. https://doi.org/10.3390/en12173229
Ling J, Xu Y, Lu C, Lai W, Xie G, Zheng L, Talawar MP, Du Q, Li G. Enhancing Stability of Microalgae Biocathode by a Partially Submerged Carbon Cloth Electrode for Bioenergy Production from Wastewater. Energies. 2019; 12(17):3229. https://doi.org/10.3390/en12173229
Chicago/Turabian StyleLing, Jiayin, Yanbin Xu, Chuansheng Lu, Weikang Lai, Guangyan Xie, Li Zheng, Manjunatha P. Talawar, Qingping Du, and Gangyi Li. 2019. "Enhancing Stability of Microalgae Biocathode by a Partially Submerged Carbon Cloth Electrode for Bioenergy Production from Wastewater" Energies 12, no. 17: 3229. https://doi.org/10.3390/en12173229