Abstract
Reconstructing pH from biogenic carbonates using boron isotopic compositions relies on the assumption that only borate and no boric acid, is present. Red coralline algae are frequently used in palaeoenvironmental reconstruction due to their widespread distribution and regular banding frequency. Prior to undertaking pH reconstructions using red coralline algae we tested the boron composition of the red coralline alga Lithothamnion glaciale using high field NMR. In bulk analysed samples, thirty percent of boron was present as boric acid. We suggest that prior to reconstructing pH using coralline algae 1) species-specific boron compositions and 2) within-skeleton special distributions of boron are determined for multiple species. This will enable site selective boron analyses to be conducted validating coralline algae as palaeo-pH proxies based on boron isotopic compositions.
Similar content being viewed by others
Introduction
Concerns about current and projected ocean acidification as a consequence of oceanic uptake of anthropogenic atmospheric CO21, heighten our need to understand past oceanic pH. Boron isotopic composition (δ11B) of marine biogenic carbonates has been considered an attractive pH proxy for several decades since the early assessments using foraminifera2,3. As a marine pH proxy, δ11B has already provided insight using several marine biomineralisers including much further work on foraminifera4,5,6,7,8,9, corals10,11,12,13,14 and brachiopods15,16,17.
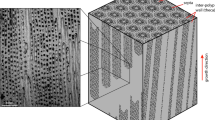
Free-living (maerl / rhodoliths) and encrusting (crustose coralline algae) red coralline algae are attractive marine pH proxy organisms since they have a global pole to pole distribution providing an outstanding spatial data source that spans a range of water temperatures and salinities. In addition, the growth mechanism of coralline algae whereby new growth occurs on top of old provides a rich temporal record18,19. The high Mg-calcite of coralline algae records ambient seawater temperature18,20 with the seasonal growth bands (Figure 1) providing the potential for a pH calendar.
In the ocean, boron exists in two molecular species: trigonal boric acid (B(OH)3) and tetrahedral borate (B(OH)-4). From here on in, boric acid and borate are referred to as B3 and B4 respectively. The ratio of B3 to B4 is sensitive to pH with B3 being more abundant at low pH2,6,8,21. There are two isotopes of B: 11B and 10B with 11B being four times as abundant as 10B. The isotopic fractionation between 11B and 10B in the dissolved species can be represented by the following mass balance:

Boric acid is enriched in 11B relative to borate ion by 27.2‰22. As proportions of B(OH)3 and B(OH)-4 vary, so will the isotopic composition such that δ11B in marine carbonates is a function of pH, assuming that only B(OH)-4 is incorporated into marine carbonates2. For any biomineral proxy organism, this sole incorporation of B4 is the key assumption to be verified23,24. In the case of coralline algae the combination of high resolution spatial and temporal data cannot be exploited until this assumption is checked. If it is the case that only B4 is incorporated into the coralline algal skeleton then the B/Ca ratio and δ11B may be used to determine past ocean pH in coralline algae as in some species of planktonic foraminifera4 or in coral skeleton25,26. 11B magnetic angle spinning nuclear magnetic resonance (MAS NMR) has proved to be a powerful probe of boron speciation in biogenic carbonates10,27. Compared to previous studies, the experiments reported here have been performed at very high magnetic field (18.8 T), leading to highly resolved spectra with well-separated B3 and B4 regions. Moreover, the experiments have been conducted on a prototype measurement probehead equipped with a B-free stator, leading to 11B background free spectra. For the first time, the combination of high field and 11B background free spectra allows the investigation of the 11B speciation in biogenic carbonate with efficient and standard single pulse acquisitions. The B3/B4 quantification was then derived from standard signal integration whereas previous studies required 11B background signal subtraction10 or complex signal simulation27 that introduced error in the B3/B4 ratio determination. Thus, high field MAS NMR is applied here to red coralline algae as a primary assessment of the suitability of this globally distributed biogenic carbonate as a marine pH recorder.
Results
MAS NMR performed at high field (18.8 T) readily resolves the B3 and B4 signals in L. glaciale (Figure 2). In addition to the main B3 (18 ppm) and B4 (2 ppm) signals, a third resonance at 6 ppm can be observed, indicating that a second B4 species is present at low amount inside the samples. In L. glaciale with and without epithelium present, boron in the trigonal form (B3) accounts for around 30% of the boron present and B3 is thus present in the calcified high Mg skeleton (Table 1). Boron in the tetragonal forms (B4) account for around 70% with 64% originate from the major B4 unit at 2 ppm and 6% from the minor B4 unit at 6 ppm. This finding indicates that the assumption of only tetrahedral boron (B4) being incorporated into biogenic carbonates required for pH reconstruction is not met on a whole-branch scale in coralline algae, since it is clear that a significant amount of trigonal boron (B3) is present. This incorporation of a significant proportion of B3 has occurred in L. glaciale from Loch Sween where the mean annual pH is 8.10. In these conditions, the proportion of B3 to B4 in seawater is around 4:16,817. Thus, the proportion of B3 in L. glaciale does not reflect the direct proportion of B3 relative to B4 in seawater, B4 being majority incorporated into the skeleton. Importantly, lower pH in ocean acidification conditions would increase the proportion of B3 to B4 in seawater.
Discussion
A primary assessment of the pH-reconstruction assumption that boron is incorporated into coralline algae solely in the tetrahedral (B4) form was made using powdered whole branches. Both trigonal and tetrahedral boron were present; had high-field NMR indicated that all boron was in the tetrahedral form, then L. glaciale could then be used in bulk powdered form to determine past oceanic pH. Boric acid is not localised just in the epithelium, but is present in the high Mg skeleton as well. The presence of B3 was already observed in corals and in foraminifera9,19. This presence could account for some of the vital effects in the boron isotopic compositions of biocarbonates, together with an elevation of pH at the sites of calcification28. Indeed, some species of coralline algae can increase their internal pH up to 0.5 pH units in light conditions29 It is clear that a bulk approach cannot be pursued for pH reconstruction and likely indicates that spatially non-selective analysis of within growth band CaCO3 may also be sampling both B3 and B4. To account for the presence of B3, it is possible to adjust calculations allowing for a 30% B3 presence. However, prior to this, four further checks should be conducted:
-
1
Test boron composition in other coralline algal species to determine whether L. glaciale portrays the exception or the rule.
-
2
Skeletal spatially-specific testing of L. glaciale and other coralline algal species investigating winter and summer growth bands separately since the faster growth rate of summer growth and slower winter growth may result in different rates of B3 incorporation. Faster growth rates in summer occur in tandem with more Mg incorporation18 and this could be associated with more or less B3 incorporation. Slow winter growth may exclude B3 and thus the calcite deposited during winter may provide a more ideal pH proxy. Understanding this is critical in the light of observations in other organisms where B3 incorporation is not uniform throughout biomineral structures e.g. as in corals10.
-
3
Quantification of B3 incorporation in L. glaciale grown in different pH environments to determine any pH-depended variation in B3 incorporation to determine whether B3 is incorporated directly into the skeleton or if B4 is modified to B3 after boron incorporation as suggested by27.
-
4
Measurement of δ11B to test if B3 is incorporated from seawater or is due to a change of B4 after the carbonate precipitation as proposed by27.
Species-specific boron compositions and within-skeleton special distributions of boron are determined for multiple species of coralline algae. This will enable site selective boron analyses to be conducted validating coralline algae as palaeo-pH proxies based on boron isotopic compositions.
Methods
Duplicate specimens of a single species of free-living red coralline algae, Lithothamnion glaciale were collected from Loch Sween, Scotland 56°01.99'N, 05°36.13'W from 5 m depth using SCUBA. Annual temperature range at the collection site is 6–17°C and average pH is 8.10 (measured in situ with YSI EXO2 Sonde equipped with pH probe which was calibrated monthly and drift adjusted).
Branches of two air-dried specimens were brushed to remove any ground in material and powdered using a Retsh MM400 ball mill. One thallus was crushed with the outer epithelium in place while the second thallus had the epthelium removed using a sanding drill (Dremmel Tool) prior to crushing.
11B magic angle spinning nuclear magnetic resonance (MAS-NMR) experiments were carried out at 256.71 MHz on a Bruker Avance III spectrometer. A 3.2 mm probehead, operating at a spinning frequency of 20 kHz and equipped with a Vespel® stator (avoiding the presence of probe background signal in the11B NMR experiments) has been used. The spectra were acquired with 0.5 μs pulse length (10° pulse angle) and 700 k transients separated by a 0.2 s recycle delay, leading to a complete measuring time of 40 hours. The trigonal (B3) to tetrahedral (B4) ratios were directly extracted from the spectra by standard signal integration and errors were determined from noise signal integration.
References
Orr, J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686, 10.1038/nature04095 (2005).
Hemming, N. G. & Hanson, G. N. Boron isotopic composition and concentration in modern marine carbonates. Geochim Cosmochim Acta 56, 537–543, 10.1016/0016-7037(92)90151-8 (1992).
Spivack, A. J., You, C. F. & Smith, H. J. Foraminiferal boron isotope ratios as a proxy for surface ocean pH over the past 21 Myr. Nature 363, 149–151, 10.1038/363149a0 (1993).
Yu, J., Elderfield, H. & Hoenisch, B. B/Ca in planktonic foraminifera as a proxy for surface seawater pH. Paleoceanography 22 Pa220210.1029/2006pa001347 (2007).
Kasemann, S. A., Schmidt, D. N., Bijma, J. & Foster, G. L. In situ boron isotope analysis in marine carbonates and its application for foraminifera and palaeo-pH. Chem Geol 260, 138–147, 10.1016/j.chemgeo.2008.12.015 (2009).
Yu, J., Foster, G. L., Elderfield, H., Broecker, W. S. & Clark, E. An evaluation of benthic foraminiferal B/Ca and delta B-11 for deep ocean carbonate ion and pH reconstructions. Earth Planet Sci Lett 293, 114–120, 10.1016/j.epsl.2010.02.029 (2010).
Rollion-Bard, C. & Erez, J. In situ boron isotopic measurements in cultured foraminifera: Implications for delta B-11 vital effects. Geochim Cosmochim Acta 74, A879–A879 (2010).
Rae, J. W. B., Foster, G. L., Schmidt, D. N. & Elliott, T. Boron isotopes and B/Ca in benthic foraminifera: Proxies for the deep ocean carbonate system. Earth Planet Sci Lett 302, 403–413, 10.1016/j.epsl.2010.12.034 (2011).
Henehan, M. J. et al. Calibration of the boron isotope proxy in the planktonic foraminifera Globigerinoides ruber for use in palaeo-CO2 reconstruction. Earth Planet Sci Lett 364, 111–122, 10.1016/j.epsl.2012.12.029 (2013).
Rollion-Bard, C. et al. Boron isotopes as pH proxy: A new look at boron speciation in deep-sea corals using 11B MAS NMR and EELS. Geochim Cosmochim Acta 75, 1003–1012, 10.1016/j.gca.2010.11.023 (2011).
Dissard, D. et al. Light and temperature effects on δ11B and B/Ca ratios of the zooxanthellate coral Acropora sp.: results from culturing experiments. Biogeosciences 9, 4589–4605, 10.5194/bg-9-4589-2012 (2012).
Anagnostou, E., Huang, K. F., You, C. F., Sikes, E. L. & Sherrell, R. M. Evaluation of boron isotope ratio as a pH proxy in the deep sea coral Desmophyllum dianthus: Evidence of physiological pH adjustment. Earth Planet Sci Lett 349, 251–260, 10.1016/j.epsl.2012.07.006 (2012).
Reynaud, S., Hemming, N. G., Juillet-Leclerc, A. & Gattuso, J. P. Effect Of pCO(2) and temperature on the boron isotopic composition of the zooxanthellate coral Acropora sp. Coral Reefs 23, 539–546, 10.1007/s00338-004-0399-5 (2004).
Honisch, B. et al. Assessing scleractinian corals as recorders for paleo-pH: Empirical calibration and vital effects. Geochim Cosmochim Acta 68, 3675–3685, 10.1016/j.gca.2004.03.0026 (2004).
Joachimski, M. M., Simon, L., van Geldern, R. & Lecuyer, C. Boron isotope geochemistry of Paleozoic brachiopod calcite: Implications for a secular change in the boron isotope geochemistry of seawater over the Phanerozoic. Geochim Cosmochim Acta 69, 4035–4044, 10.1016/j.gca.2004.11.017 (2005).
Penman, D. E., Hoenisch, B., Rasbury, E. T., Hemming, N. G. & Spero, H. J. Boron, carbon and oxygen isotopic composition of brachiopod shells: Intra-shell variability, controls and potential as a paleo-pH recorder. Chem Geol 340, 32–39, 10.1016/j.chemgeo.2012.11.016 (2013).
Lecuyer, C., Grandjean, P., Reynard, B., Albarede, F. & Telouk, P. B-11/B-10 analysis of geological materials by ICP-MS Plasma 54: Application to the boron fractionation between brachiopod calcite and seawater. Chem Geol 186, 45–55, Pii s0009-2541(01)00425-910.1016/s0009-2541(01)00425-9 (2002).
Kamenos, N. A., Cusack, M. & Moore, P. G. Coralline algae are global palaeothermometers with bi-weekly resolution. Geochimica et Cosmochimica Acta. 72, 771–779 (2008).
Kamenos, N. A. North Atlantic summers have warmed more than winters since 1353 and the response of marine zooplankton. Proceedings of the National Academy of Sciences 107, 22442–22447, 10.1073/pnas.1006141107 (2010).
Darrenougue, N., De Deckker, P., Payri, C., Eggins, S. & Fallon, S. Growth and chronology of the rhodolith-forming, coralline red alga Sporolithon durum. Mar Ecol Prog Ser 474, 105-119, 10.3354/meps10085 (2013).
Hershey, J. P., Fernandez, M., Milne, P. J. & Millero, F. J. The Ionization of Boric Acid in NaCl, Na-Ca-Cl AND Na-Mg-Cl Solutions at 25 Degrees C. Geochim Cosmochim Acta 50, 143-148, 10.1016/0016-7037(86)90059-1 (1986).
Klochko, K., Kaufman, A. J., Yao, W., Byrne, R. H. & Tossell, J. A. Experimental measurement of boron isotope fractionation in seawater. Earth Planet Sci Lett 248, 276–285, 10.1016/j.epsl.2006.05.034 (2006).
Xiao, J., Jin, Z. D., Xiao, Y. K. & He, M. Y. Controlling factors of the delta B-11-pH proxy and its research direction. Environmental Earth Sciences 71, 1641–1650, 10.1007/s12665-013-2568-8 (2014).
Pagani, M., Lemarchand, D., Spivack, A. & Gaillardet, J. A critical evaluation of the boron isotope-pH proxy: The accuracy of ancient ocean pH estimates. Geochim Cosmochim Acta 69, 953–961, 10.1016/j.gca.2004.07.029 (2005).
Liu, Y. et al. Instability of seawater pH in the South China Sea during the mid-late Holocene: Evidence from boron isotopic composition of corals. Geochim Cosmochim Acta 73, 1264–1272, 10.1016/j.gca.2008.11.034 (2009).
Douville, E. et al. Abrupt sea surface pH change at the end of the Younger Dryas in the central sub-equatorial Pacific inferred from boron isotope abundance in corals (Porites). Biogeosciences 7, 2445–2459, 10.5194/bg-7-2445-2010 (2010).
Klochko, K., Cody, G. D., Tossell, J. A., Dera, P. & Kaufman, A. J. Re-evaluating boron speciation in biogenic calcite and aragonite using B-11 MAS NMR. Geochim Cosmochim Acta 73, 1890–1900, 10.1016/j.gca.2009.01.002 (2009).
Rollion-Bard, C., Chaussidon, M. & France-Lanord, C. Biological control of internal pH in scleractinian corals: Implications on paleo-pH and paleo-temperature reconstructions. Comptes Rendus Geoscience 343, 387–396 (2011).
Hurd, C. L. et al. Metabolically induced pH fluctuations by some coastal calcifiers exceed projected 22nd century ocean acidification: a mechanism for differential susceptibility? Global Change Biology 17, 3254–3262, 10.1111/j.1365-2486.2011.02473.x (2011).
Acknowledgements
We thank Stephen Wimperis and Smita Odedra of the School of Chemistry, University of Glasgow for carrying out preliminary NMR analyses. GT acknowledges the financial support of TGIR-RMN-THC Fr3050 CNRS.
Author information
Authors and Affiliations
Contributions
M.C. and N.A.K. conceived the project, N.K. prepared the samples, C.R.-B. And G.T. conducted the NMR analyses, N.K. prepared Figure 1 and G.T. prepared Figure 2, M.C. wrote the main manuscript and all authors contributed to the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Cusack, M., Kamenos, N., Rollion-Bard, C. et al. Red coralline algae assessed as marine pH proxies using 11B MAS NMR. Sci Rep 5, 8175 (2015). https://doi.org/10.1038/srep08175
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08175
This article is cited by
-
First freshwater coralline alga and the role of local features in a major biome transition
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.