Abstract
Dispersal and reproductive traits of successful plant invaders are expected to undergo strong selection during biological invasions. Numerous Asteraceae are invasive and display dimorphic fruits within a single flower head, resulting in differential dispersal pathways - wind-dispersed fruits vs. non-dispersing fruits. We explored ecotypic differentiation and phenotypic plasticity of seed output and fruit dimorphisms in exotic Chilean and native Spanish populations of Leontodon saxatilis subsp. rothii. We collected flower heads from populations in Spain and Chile along a rainfall gradient. Seeds from all populations were planted in reciprocal transplant trials in Spain and Chile to explore their performance in the native and invasive range. We scored plant biomass, reproductive investment and fruit dimorphism. We observed strong plasticity, where plants grown in the invasive range had much greater biomass, flower head size and seed output, with a higher proportion of wind-dispersed fruits, than those grown in the native range. We also observed a significant ecotype effect, where the exotic populations displayed higher proportions of wind-dispersed fruits than native populations. Together, these patterns reflect a combination of phenotypic plasticity and ecotypic differentiation, indicating that Leontodon saxatilis has probably increased propagule pressure and dispersal distances in its invasive range to enhance its invasiveness.
Similar content being viewed by others
Introduction
The impacts of invasive plant species on resident communities and ecosystem functions are a global concern, which has led to considerable resources being invested into studying invasiveness. Of particular importance is predicting which plants will become invasive. The invasiveness of alien plants depends on the habitat characteristics of the recipient area (e.g. the fluctuating resource availability theory1), as well as on species traits2. The characteristics of recipient habitats have received considerable attention3, 4, highlighting that the habitats more prone to be invaded are those that are more productive or more disturbed5, 6. However, despite some recent attention, understanding the role played by species traits in the invasion process still remains a key knowledge gap in invasion biology7, 8.
These knowledge gaps exist because invasion success is primarily studied species-by-species9, 10, but these gaps also exist because most studies do not compare species traits in native and invasive ranges11. Nevertheless, some plant traits related to reproductive and dispersal characteristics have been suggested to be of key importance to invasiveness, such as plant growth rate, seed size, and distance of seed dispersal7, 12,13,14. For example, previous studies have shown that greater plant growth accounts for the invasiveness of many alien plant species15. Likewise, there is a correlation between seed size and greater invasiveness, where species with numerous smaller seeds have higher abundances and numbers of sites occupied than species with larger seeds7, 16.
Distance of seed dispersal appears to be positively related with both spread rate and geographic range size of invasive plants17. In order to successfully colonize new environments, plants largely rely on two main strategies: enhancing seed output and/or increasing the distance of seed dispersal18, 19. Greater seed output is determined by the fraction of total biomass allocated to reproduction20, 21. Increasing the distance of seed dispersal results from selection acting on the dispersal traits or due to maternal effects22, 23.
The dispersal syndrome of a plant can be inferred from the morphology of its propagules24. Plants use polymorphic seeds and fruits to overcome different microhabitats and environmental conditions25, allowing the colonization of highly unpredictable habitats26, 27. Therefore, it can be expected that seed output and distance of seed dispersal are likely to undergo strong positive selection during plant invasions28, 29, although more studies are needed to elucidate the roles of plasticity and selection acting on these traits30, 31.
Many invasive plant species are Asteraceae32, and interestingly, many Asteraceae also present fruit (i.e. achene) dimorphisms (i.e. heterocarpy26). Leontodon saxatilis subsp. rothii Maire is a daisy common in the Mediterranean Basin. It is native and typical of Spanish grasslands, although it is now widely naturalized in Chile, plus other Mediterranean regions such as California33 and southern Australia34. Leontodon saxatilis constitutes an ideal candidate to study the importance and evolution of dispersal traits during invasions because it produces two morphologically distinct fruits and it has recently undergone a successful invasion. It produces peripheral fruits that lack dispersal structures, plus central fruits that are dispersed by wind.
The aim of this study was to explore plasticity and ecotypic differences in seed output and fruit dimorphism patterns associated with the colonization of L. saxatilis into Chile. We collected L. saxatilis fruits from 5 populations in its native range in Spain and 4 populations in its invasive range in Chile. We grew these plants in common garden trials at both its origin and its invasive range, and quantified the ecotypic and plastic components of variation in reproductive and dispersal-related traits. An ecotype effect would be observed where the geographic origin of plants explains differences in reproductive and dispersal-related traits at the same common garden trial. Similarly, phenotypic plasticity would be observed where individuals from the same geographic origin produce different reproductive and dispersal-related trait values in the two common garden trials. We expected that plants grown in the invasive range would be more productive because of reduced competition and enemy release. Similarly, we expected that plants from the invasive range would be more productive because, during invasion, it is likely that selection occurred on traits that allowed plants to undergo longer distance seed dispersal and produce greater amounts of seed.
Results
We use the term biomass to refer to above-ground biomass, number of fruits per flower head to refer to average number of fruits per flower head, seed output per plant to refer to total estimated seed output per plant, and proportion of central wind dispersed fruits to refer to average proportion of central fruits within a flower head. Likewise, we use the simplified names of the variables even though some of them were transformed to carry out the analyses, with details of transformations in the methods. In the case of predictor variables, Site represents the country of reciprocal transplant trials (Chile or Spain), whereas Origin corresponds to the country of source populations (Chile or Spain). According to the linear models, both Site and Origin explained most of the variation of our response variables.
Plant growth
Planting site had the largest effect on biomass, with larger plants in Chile (mean biomass in Chile = 25.8 g ± 1.19 SE; in Spain = 6.7 g ± 0.3 SE; P < 0.001; Table 1; see Supplementary Table S1 online for detailed information). To a lesser extent, biomass was also influenced by precipitation of the source population. The site*precipitation effect was also significant. For populations grown in Chile, there was a trend of larger biomass for populations from lower rainfall areas (r = 0.361; P < 0.001), whereas for populations grown in Spain, precipitation and biomass were not correlated (r = 0.036; P > 0.05).
Reproductive investment
Variation in all three fitness traits related to reproductive investment (i.e. number of flower heads per plant, number of fruits per flower head, and seed output per plant) was largely explained by planting site, being greater for the three variables in the Chilean common garden than in the Spanish one. The country of origin of the populations also had a significant but weak effect on the number of flower heads per plant and the number of fruits per flower head with higher values for the populations coming from Chile.
All the variables reflecting reproductive investment were significantly and positively correlated with biomass (Fig. 1a–c), regardless of the planting site and the origin of the population (number of flower heads per plant: r2 = 0.7–0.8; number of fruits per flower head: r2 = 0.5–0.6 but only for the common garden in Spain; seed output: r2 = 0.8–0.9). The regression slopes between the number of fruits per flower head and biomass were ten times steeper in Spain than in Chile, regardless of the country of origin of populations (3.37 and 2.84 for Chilean and Spanish populations grown in Spain, and 0.30 and 0.27 for Chilean and Spanish populations grown in Chile, respectively).
Relationships between biomass and (a) number of flower heads per plant, (b) number of fruits per flower head and (c) seed output per plant at each site. Close circles represent Chilean populations (Ch) whereas open ones refer to Spanish ones (S). Significant relationships are shown by discontinuous (Chilean populations) or continuous (Spanish populations) lines. For each relationship, regression coefficient and its significance are shown (* < 0.05; ** < 0.01; *** < 0.001).
Dispersal strategy
Both central (wind-dispersed) and peripheral (non-dispersing) fruits increased with the number of fruits per flower head in populations from both countries grown in the native and the invaded ranges, although this trend was especially noticeable for central fruits (central fruit regression coefficients r2 > 0.9 in both sites; peripheral fruit regression coefficients r2 0.2 to 0.5 in Chile and r2 0.4 to 0.7 in Spain) (Fig. 2a,b).
Relationships between (a) number of central wind-dispersed and (b) peripheral non-dispersing fruits and the number of fruits per flower head at each site. Close circles represent Chilean populations (Ch) whereas open ones refer to Spanish ones (S). Significant relationships are shown by discontinuous (Chilean populations) or continuous (Spanish populations) lines. For each relationship, regression coefficient and its significance are shown (* < 0.05; ** < 0.01; *** < 0.001).
The proportion of central fruits was significantly higher in the Chilean common garden, although the country of origin of the populations also explained this variable (Table 1). Chilean populations always had a higher proportion of central fruits than Spanish populations (mean proportion of central fruits: in Chile = 0.87 ± 0.004 SE, in Spain = 0.81 ± 0.005 SE; mean proportion of central fruits: for Chilean populations = 0.87 ± 0.003 SE, for Spanish populations = 0.80 ± 0.006 SE).
Discussion
The importance of comparing invasive plant performance in reciprocal transplant trials across native and invasive ranges has only recently been emphasized35. Our study represents one of the first to track key invasive traits via reciprocal transplant trials established in both the native and invasive range of a widely distributed and impactful invasive species36,37,38. We showed that the most common alien plant species in Chile, the daisy Leontodon saxatilis, combined multiple strategies in being a successful invader from its native Spanish range into Chile (a combination that is consistent with that observed for other species39). It displayed strong phenotypic plasticity in reproductive investment (i.e. number of flower heads produced, number of fruits per flower head, and seed output) and in the proportion of central wind-dispersed fruits (i.e. the reproductive investment and the proportion of wind-dispersed fruits), which were both greater in the invasive range than in the native one. This species also displayed strong ecotypic differentiation for the proportion of central wind-dispersed fruits, and significant but weaker ecotypic effects for the number of flower heads per plant and the number of fruits per flower head. These trends suggest that L. saxatilis has undergone strong selection for bigger flower heads and longer distance seed dispersal. In addition, L. saxatilis displayed greater growth potential in the novel environment, possibly as a result of fewer competitors (see examples of enemy release40). In combination, these traits should increase the invasiveness of L. saxatilis by increasing propagule pressure and the distance of seed dispersal in its invasive range.
The residence time hypothesis proposes that the longer an alien species is present in a new environment, the higher the chances that it will become a successful colonizer41, 42. The first record of L. saxatilis in Chile was 196343. Due to its short residence time, a narrow regional distribution of this species in Chile would be expected43,44,45. However, since its arrival, it has become widely distributed (from 32°S to 42°S) and is present in several administrative regions far beyond the Mediterranean area46, indicating an unexpectedly high invasiveness. L eontodon saxatilis is the most frequent alien species in Chile47, which provides further evidence of its success in colonizing new areas and overcoming a diversity of novel conditions48. Anthropogenic activities seem to determine the spatial distribution of L. saxatilis in its invasive range49. The colonization ability of this species depends mostly on its capacity to colonize areas created after human disturbance (in line with the ‘novel niche hypothesis’50) and its great dispersal ability (in line with the ‘propagule pressure hypothesis’51).
Once L. saxatilis arrives into an exotic location it most likely follows a combined bet-hedging strategy52, 53. Central wind-dispersed fruits are always higher in number and spread further than peripheral fruits. However, wind-dispersal is a high-risk strategy (i.e. wind-dispersed fruits have a high probability of falling in unfavourable sites), and is strongly affected by stochastic events. The peripheral fruits lack dispersal structures and rely on a low risk strategy. Peripheral fruits are larger, with a thicker pericarp and are viable for longer than central fruits54, 55. Peripheral fruits rely on a self-replacement strategy56, being most able to colonize the location where their mother plant grew57. The low risk, self-replacement strategy increases the chances of peripheral fruits supporting the naturalization of L. saxatilis in the vicinity of the mother plant, which allows it to overcome the challenges of the ‘novel niche hypothesis’58. Conversely, the high risk strategy allows wind-dispersed fruits to achieve longer distance seed dispersal and thus reach areas further afield from mother plants57, 59. This longer distance of seed dispersal would help expand the distribution of this species and overcome the challenges related to the ‘propagule pressure hypothesis’.
We demonstrated that the plant growth and reproductive investment of L. saxatilis had strong phenotypic plasticity and, to a lesser extent, ecotypic differentiation. Leontodon saxatilis biomass was far greater when it grew in its invasive range than when it grew in its native range, which is similar to other alien species60,61,62,63.
Biomass produced by this species was invested in maximizing reproduction, enhancing the two fitness parameters involved in seed output – the number of flower heads produced per plant, and the number of fruits produced per flower head. Seed output displayed by L. saxatilis was highly dependent on biomass, which also showed strong phenotypic plasticity. Seed output was significantly greater for individuals grown in their invasive range, constituting an effective strategy for this species to produce more dispersal units and promote its propagule pressure in a novel environment.
The number of fruits produced by L. saxatilis affects its dispersal ability. We showed that fitness increased with biomass, however the number of fruits produced per flower head had an asymptotic response as a consequence of an upper limit in the size that the flower head could reach. The number of fruits per flower head in the invasive range was greater than in the native range, however the relationship between the number of fruits per flower head and biomass was significant but non-linear. The weak linear association between the number of fruits per flower head and biomass indicates that the flower head has architectural constraints. In Asteraceae flower head size is restricted by the receptacle (i.e. the surface of the flower head where the fruits are implanted) and can only increase up to a limit that characterizes each species.
We showed that all plants in our study increased the number of central wind-dispersed and peripheral non-dispersing fruits with the number of fruits per flower head. However, this relationship was stronger for central fruits than for peripheral fruits (central fruits: r2 > 0.9; peripheral fruits r2 = 0.2–0.7), and the regression slope between central fruits and biomass was an order of magnitude greater than the regression slope for peripheral fruits and biomass. Considering that the number of fruits per flower head is a surrogate for the size of flower heads (i.e. it is limited by the surface of the receptacle), the different performance of the two types of fruits is again likely to be due to architectural constrictions. When the radius of the receptacle increases, the number of peripheral fruits (arranged in one row at the edge in the involucral bracts) increases with the length of the circumference, whereas the number of central fruits increases with the surface area of the receptacle. Therefore, the carrying capacity of the flower head differs for each type of fruit. As the number of fruits per flower head was significantly higher for populations grown in the exotic site, more central wind-dispersed fruits were produced in the invasive range.
The proportion of wind-dispersed fruits produced within a flower head also presented greater phenotypic plasticity for individuals grown in the invasive range. Nevertheless, the proportion of wind-dispersed fruits mostly presented ecotypic differentiation, where variation in the proportion of wind-dispersed fruits was best explained by the country of origin of populations. The proportion of wind-dispersed fruits was significantly greater in Chilean populations than in Spanish ones, which suggests that selection for longer-dispersal ability had occurred in Chile. Ecotypic differentiation has also been reported for other Asteraceae, such as Crepis sancta 64. Having a greater proportion of wind-dispersed fruits results in maximizing seed dispersal in the colonized area, thus the invasiveness of L. saxatilis appears to be greater for exotic populations and for individuals grown in its invasive range.
In summary, we show that colonization ability is an important component of a plant’s invasiveness. Some invasive plant species, such as the daisy L. saxatilis studied here, appear to combine phenotypic plasticity and ecotypic differentiation as coadaptations to cope with the novel conditions of the invasive range and to increase propagule pressure and distance of seed dispersal in its new environment. Leontodon saxatilis, the most frequently observed species in central Chile, seems to only require simple mechanisms to increase its capacity of invasive expansion. A combination of simple architectural relationships, largely dependent on biomass (i.e. the number and size of flower heads), and different dispersal strategies (i.e. wind vs. non-dispersing) impact on the invasiveness of L. saxatilis in its invasive range. Alien species with fruit dimorphisms should be carefully controlled and their spread monitored, with daisies requiring special attention due to their great invasive potential. Consequently, studying the evolution of dispersal abilities of invasive species is important for understanding invasiveness and therefore management of biological invasions and conservation policies.
Methods
Study species
A large proportion of species from the Mediterranean Basin were introduced to Chile during the Spanish conquest of Latin America and many of these species have become naturalized in the Mediterranean climatic region of central Chile. The percentage of invasive species in central Chile that belong to the Asteraceae family is ca. 13.9%47. Most daisies produce two types of fruits that occupy different locations on the flower head. Fruits are either on the periphery or in the center, and they show different morphologies, germination requirements and dispersal abilities65, 66. Central fruits are generally smaller, lighter and have structures that allow wind-dispersal (anemochory). Peripheral fruits are larger, heavier and lack specific structures for wind-dispersal67, 68, but often have modifications that make them suitable for animal (passive-zoochory66) or water dispersal (hydrochory67).
Leontodon saxatilis subsp. rothii produces dark brown heavy fruits without a pappus or other specific dispersal structures and light brown fruits with a pappus that are wind-dispersed. Leontodon saxatilis subsp. rothii is the accepted name of the species although it has many recognized synonyms (e.g. Colobium hispidum Roth, Leontodon longirostris (Finch & P.D. Sell) Talavera, Leontodon saxatilis subsp. hispidus (Roth) Castrov. & M. Laínz, Leontodon saxatilis subsp. longirostris (Finch & P.D. Sell) P. Silva, Leontodon taraxacoides subsp. hispidus (Roth) Kerguélen, Leontodon taraxacoides subsp. longirostris Finch & P.D. Sell, Thrincia hispida (Roth) Roth, Thrincia saxatilis subsp. hispida (Roth) Holub & Moravec69, 70).
Study area
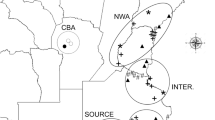
Our study was based in the Mediterranean grasslands of Chile and Spain. In Chile, populations of L. saxatilis were collected in the central region (from 32°31′ to 37°00′ S and 70°46′ to 72°34′ W), with mean annual precipitation between 300 and 1200 mm. In Spain, the populations were collected in the center-west of the Iberian Peninsula (from 37°51′ to 40°14′N and from 4°23′ to 7°02′W), with mean annual precipitation between 400 and 1100 mm (Fig. 3). Both regions are similar in terms of lithology (acid substrate, derived from igneous or metamorphic rocks), plant physiognomy (the structure consists of a continuous herbaceous layer with scattered trees), and land use (mainly extensive livestock grazing by sheep and cattle). Almost half of the species present in the Mediterranean Chilean grasslands are aliens that largely originated in the Mediterranean Basin47, and the Iberian Peninsula71.
Map of the studied areas of Mediterranean grasslands in Spain and Chile, including sampling sites (see Table 2). Grey tones represent rainfall variability in each country. The locations of the reciprocal transplant trials are shown (x). The figure and the maps were created manually using Microsoft PowerPoint version 14.0.7166.5000 by modifying images from Google Maps (Microsoft Office Professional Plus 2010; https://microsoft-office-professional-plus.uptodown.com/windows; map of Chile (https://www.google.es/maps/place/Chile/@-35.3617722,-89.1181162,4z/data=!3m1!4b1!4m5!3m4!1s0×9662c5410425af2f:0×505e1131102b91d!8m2!3d-35.675147!4d-71.542969 (Map Data ©2016 Google, INEGI)); map of Spain (https://www.google.es/maps/place/Espa%C3%B1a/@40.1300278,-8.2052927,6z/data=!3m1!4b1!4m5!3m4!1s0xc42e3783261bc8b:0xa6ec2c940768a3ec!8m2!3d40.463667!4d-3.74922 (Map Data ©2016 Google)).
Data collection
We selected populations of L. saxatilis representative of the rainfall gradient existing in the Mediterranean regions of Spain and Chile. Five populations were sampled in Spain, and four in Chile (Fig. 3; Table 2). We collected mature flower heads from 50 individuals of L. saxatilis for each population in spring of 2010 (May and June in Spanish sites, and October and November in Chilean sites). Geographic coordinates, altitude and climate conditions including annual precipitation and mean annual temperature were recorded at each site (Table 2). Climate data were obtained from the State Meteorological Agency (AEMET, http://www.aemet.es) and the Atlas Climático Digital de la Península Ibérica 72 for Spain, and from WorldClim73 for Chile.
Flower heads were cleaned in the lab to obtain fruits. Peripheral fruits were chosen for subsequent planting because of their greater success in pre-germination studies (see previous studies57, 68). Seeds from each population were germinated in petri dishes and then transplanted into subplots within two common garden trials (hereafter sites). Sites were the Experimental Centre of Cauquenes-INIA, Chile (35°58′ S, 72°17′ W; 140 m a.s.l.; 14.4 °C; 748 mm of mean annual precipitation) and the Faculty of Agronomy of the Polytechnic University of Madrid, Spain (40°26′N, 3°44′W; 600 m a.s.l.; 15 °C of mean annual temperature; 484 mm. of mean annual precipitation). The conditions within both common gardens were controlled so there was no herbivory or competition that could affect the experiment results. Ideally, including replicates of common garden experiments within each country would have been desirable to explore the effect of site74, but in this case it was not possible due to logistical and bioethical restrictions.
Planting was conducted in June 2012 in Chile and October 2012 in Spain, and sites were prepared by removing surface vegetation. Ten and 20 seedlings of each population were planted in subplots of 100 × 50 cm long and 200 × 50 cm long in Chile and Spain, respectively. The distance between plants was 20 cm and the separation between subplots was 30 cm. A complete randomized design was used with three replicate subplots. Thus, in each common garden there were a total of 27 subplots: 15 containing populations from Spain (5 populations * 3 replicates), and 12 containing populations from Chile (4 populations * 3 replicates). The total number of individuals planted was 270 in Chile and 540 in Spain. During the period of the experiment, the amount of rainfall was similar in both sites, although slightly higher in Chile (218.3 mm in Spain and 284.8 mm in Chile). However, the temporal distribution of rainfall during this period differed for both countries, with Spain being drier in winter and more humid in spring than Chile. Throughout the experiment, 39% and 55% of individuals died in Chile and Spain, respectively, possibly influenced by these weather conditions. The final count of survivors included in our study was 166 in Chile and 245 in Spain. The fact that the individuals arranged in the reciprocal transplant trials differed between countries was due to the availability of space to carry out the experiment in both sites, which was greater in Spain than in Chile. Flower heads were collected from every individual after they were mature but before the infructescence opened, to ensure we captured all seeds.
Seed output and proportion of central (wind-dispersed) fruits were the target traits of our study because of their key role on invasiveness of L. saxatilis. When plants reached ca. 50% senescence (i.e. we observed that half of the flower heads had maturated), five flower heads were collected from each individual and traits associated with fitness and dispersal ability estimated. Plants were harvested when they reached 75% senescence.
Once all plants had been harvested, we measured aboveground biomass (hereafter biomass) and counted the number of flower heads per plant. Flower head size was measured by counting the number of fruits per flower head present in five flower heads; the central fruits were separated from the peripheral non-dispersing fruits. Then the average number of fruits per flower head was calculated. The average proportion of central fruits within a flower head was also calculated by dividing the average number of central fruits within a flower head by the average number of fruits per flower head. Finally, we estimated the total seed output per plant (i.e. the total number of seeds produced per individual) by multiplying the number of flower heads per plant by the average number of fruits per flower head.
Data analyses
We used mixed effects models to analyze the colonization ability of L. saxatilis, considering the plant individual as the unit of analysis (n = 408). Models were fitted to the following response variables: plant growth (i.e. biomass), reproductive investment (number of flower heads per plant, average number of fruits per flower head and estimated seed output per plant), and the dispersal strategy (average proportion of central wind-dispersed fruits within a flower head). Predictor variables included the site of the reciprocal transplant trials (Chile or Spain), the country of origin of the source populations (Chile or Spain), and precipitation of the source populations. The source population and the subplot where populations were planted within each common garden (subplot nested within site) were included as random effects. All the possible models including site, origin and precipitation as predictors were computed.
We used mixed effects models with a Gaussian error distribution except for the number of flower heads per plant, where we used a Poisson link function. Biomass and estimated seed output per plant were log-transformed, and precipitation was rescaled by standardization to improve model fitting. Models computed for the proportion of central fruits were weighted by the number of fruits per flower head.
We compared the possible models differing in the structure of fixed effects fit by maximum likelihood whether they had a Gaussian error distribution and the Laplace approximation when they had a Poisson error distribution. We calculated the Akaike Information Criterion corrected for small sample size (AICc)75 and selected the best-fit models (= all models with ∆AICc < 2 from the best fitting model with the lowest AICc) (See Supplementary Table S1 online for detailed information). The parsimony criterion was then applied on the subset of best models based on AICc, where the model with the lowest number of parameters was chosen for subsequent analysis. Selected models were fitted by Restricted Maximum Likelihood. Model validation of the best-fit model was based on visually assessing the normality of residuals, and we tested for model overdispersion by checking that model residual deviance was lower than the residual degrees of freedom76.
We also ran regressions between biomass and the fitness traits (number of flower heads per plant, average number of fruits per flower head, and estimated seed output per plant), and between the size of the flower head (i.e. average number of fruits per flower head) and the number of fruits of each type, central and peripheral. All regressions were run by splitting the individual plants by site and origin into four groups: (i) native populations planted in the native range, (ii) exotic populations planted in the native range, (iii) native populations planted in the invaded range, and (iv) exotic populations planted in the invaded range.
All analyses were performed in R v 3.2.377, using the base stats package plus the lme478 and AICcmodavg79 packages. Outliers were defined as data that exceeded three times the interquartile range, and these were subsequently removed prior to analyses (=1.5% of cases).
References
Davis, M., Grime, P. & Thompson, K. Fluctuating resources in plant communities: a general theory of invasibility. J. Ecol. 88, 528–534, doi:10.1046/j.1365-2745.2000.00473.x (2000).
Pyšek, P., Krivanek, M. & Jarošík, V. Planting intensity, residence time, and species traits determine invasion success of alien woody species. Ecology 90, 2734–2744, doi:10.1890/08-0857.1 (2009).
Hejda, M. et al. Invasion success of alien plants: do habitat affinities in the native distribution range matter? Global Ecol. Biogeogr. 18, 372–382, doi:10.1111/geb.2009.18.issue-3 (2009).
Kalusová, V., Chytrý, M., Kartsez, J. T., Nisihino, M. & Pyšek, P. Where do they come from and where they go? European natural habitats as donors of invasive alien plants globally. Divers. Distrib. 19, 199–214, doi:10.1111/ddi.2012.19.issue-2 (2013).
Chytrý, M. et al. Habitat invasions by alien plants: a quantitative comparison among Mediterranean, subcontinental and oceanic regions of Europe. J. Appl. Ecol. 45, 448–458, doi:10.1111/jpe.2008.45.issue-2 (2008).
Jansen, F., Ewald, J. & Zerbe, S. Ecological preferences of alien plant species in North-Eastern Germany. Biol. Inv. 13, 2691–2701, doi:10.1007/s10530-011-9939-4 (2011).
Moravcová, L., Pyšek, P., Jarošík, V. & Pergl, J. Getting the right traits: reproductive and dispersal characteristics predict the invasiveness of herbaceous plant species. PloS one 10, e0123634, doi:10.1371/journal.pone.0123634 (2015).
MacDougall, A. S. & Turkington, R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86, 42–55, doi:10.1890/04-0669 (2005).
Richardson, D. M. & Pyšek, P. Plant invasions: merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geogr. 30, 409–431, doi:10.1191/0309133306pp490pr (2006).
Pyšek, P., Jarošík, V. & Pergl, J. Alien plants introduced by different pathways differ in invasion success. PLoS One 6, e24890, doi:10.1371/journal.pone.0024890 PMID: 21949778 (2011).
van Kleunen, M., Dawson, W., Schlaepfer, D., Jeschke, J. M. & Fischer, M. Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol. Lett. 13, 947–958, doi:10.1111/j.1461-0248.2010.01503.x (2010).
Noble, I.R. Attributes of invaders and the invading process: terrestrial and vascular plants In Biological invasions: a global perspective (eds Drake, J.A. et al.). Wiley, Chichester, 301–313 (1989).
Pyšek, P. & Richardson, D.M. Traits associated with invasiveness in alien plants: where do we stand? in Biological invasions. Ecological Studies 193 (ed. Nentwig, W.) 97–126 (Springer, Berlin, 2007).
van Kleunen, M., Weber, E. & Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 13, 235–245, doi:10.1111/j.1461-0248.2009.01418.x (2010).
Grotkopp, E. & Rejmánek, M. High seedling relative growth rate and specific leaf area are traits of invasive species: phylogenetically independent contrasts of woody angiosperms. Am. J. Bot. 94, 526–532, doi:10.3732/ajb.94.4.526 (2007).
Guo, Q., Brown, J. H., Valone, T. J. & Kachman, S. D. Constraints of seed size on plant distribution and abundance. Ecology 81, 2149–2155, doi:10.1890/0012-9658(2000)081[2149:COSSOP]2.0.CO;2 (2000).
Catford, J. A. et al. Disentangling the four demographic dimensions of species invasiveness. J. Ecol. 104, 1745–1758, doi:10.1111/1365-2745.12627 (2016).
Keddy, P. & Weiher, E. Introduction: The scope and goals of research on assembly rules In Ecological assembly rules, perspectives, advances, retreats (eds Weiher, W. & Keddy, P.) 1–20 (Cambridge Univ. Press, Cambridge, UK, 1999).
Rejmánek, M., Richardson, D.M. & Pyšek, P. Plant invasions and invasibility of plant communities In Vegetation ecology Second Edition (eds van der Maarel, E. & Franklin, J.) John Wiley & Sons (Ltd, Oxford, UK, 2013).
Abrahamson, W. G. & Gadgil, M. Growth form and reproductive effort in goldenrods (Solidago, Compositae). Am. Nat. 107, 651–661, doi:10.1086/282864 (1973).
Amir, S. & Cohen, D. Optimal reproductive efforts and the timing of reproduction of annual plants in randomly varying environments. J. Theor. Biol. 147, 17–42, doi:10.1016/S0022-5193(05)80250-4 (1990).
Wheelwright, N. T. & Logan, B. A. Previous-year reproduction reduces photosynthetic capacity and slows life time growth in females of a neotropical tree. PNAS USA 101, 8051–8055, doi:10.1073/pnas.0402735101 (2004).
Fronhofer, E. A., Poethke, H. J. & Dieckmann, U. Evolution of dispersal distance: maternal investment leads to bimodal dispersal kernels. J. Theor. Biol. 365, 270–279, doi:10.1016/j.jtbi.2014.10.024 (2015).
Tiffney, B. H. Seed size, dispersal syndromes, and the rise of the angiosperms: evidence and hypothesis. Ann. Missouri Bot. Gard. 71, 551–576, doi:10.2307/2399037 (1984). Historical Perspectives of Angiosperm Evolution.
Imbert, E., Escarré, J. & Lepart, J. Achene dimorphism and among-population variations in some biological traits in Crepis sancta (Asteraceae). Int. J. Plant Sci. 157, 309–315, doi:10.1086/297350 (1996).
Harper, J.L. Population Biology of Plants. (Academic Press, London, 1977).
Cohen, D. & Levin, S. A. Dispersal in patchy environments: the effects of temporal and spatial structure. Theor. Popul. Biol. 39, 63–99, doi:10.1016/0040-5809(91)90041-D (1991).
Roze, D. & Rousset, F. Inbreeding depression and the evolution of dispersal rates: a multilocus model. Am. Nat. 166, 708–721, doi:10.1086/497543 (2005).
Ronce, O. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu. Rev. Ecol. Evol. Syst. 38, 231–253, doi:10.1146/annurev.ecolsys.38.091206.095611 (2007).
Ronce, O., Olivieri, I., Cobert, J. & Danchin, E. Perspectives on the study of dispersal evolution In Dispersal (eds Clobert, J., Danchin, E., Dhondt, A.A. & Nichols, J.D.) 123–142 (Oxford University Press, Oxford, 2001).
Pannell, J. R. Evolution of the mating system in colonizing plants. Mol. Ecol. 24, 2018–2037, doi:10.1111/mec.2015.24.issue-9 (2015).
Pyšek, P. Is there a taxonomic pattern to plant invasions? Oikos 82, 282–294, doi:10.2307/3546968 (1998).
DiTomaso, J. M. & Healy, E. A. Weeds of California and other western states 3488 (UCANR Publications: Oakland, CA, 2007).
Groves, R. H. et al. Weed categories for natural and agricultural ecosystem management. (Bureau of Rural Sciences: Canberra, 2003).
Williams, J. L., Auge, H. & Maron, J. L. Different gardens, different results: native and introduced populations exhibit contrasting phenotypes across common gardens. Oecologia 157, 239–248, doi:10.1007/s00442-008-1075-1 (2008).
Lamarque, L. J., Lortie, C. J., Porté, A. J. & Delzon, S. Genetic differentiation and phenotypic plasticity in life-history traits between native and introduced populations of invasive maple trees. Biol. Inv. 17, 1109–1122, doi:10.1007/s10530-014-0781-3 (2015).
Seipel, T. et al. Performance of the herb Verbascum thapsus along environmental gradients in its native and non‐native ranges. J. Biogeogr. 42, 132–143, doi:10.1111/jbi.2014.42.issue-1 (2015).
Gibson, A. L., Espeland, E. K., Wagner, V. & Nelson, C. R. Can local adaptation research in plants inform selection of native plant materials? An analysis of experimental methodologies. Evol. Appl. 9, 1219–1228, doi:10.1111/eva.12379 (2016).
Liao, H., D’Antonio, C. M., Chen, B., Huang, Q. & Peng, S. How much do phenotypic plasticity and local genetic variation contribute to phenotypic divergences along environmental gradients in widespread invasive plants? A meta‐analysis. Oikos 125, 905–917, doi:10.1111/oik.2016.v125.i7 (2016).
Fenner, M., Cresswell, J., Hurley, R. & Baldwin, T. Relationship between capitulum size and pre-dispersal seed predation by insect larvae in common Asteraceae. Oecologia 130, 72–77, doi:10.1007/s004420100773 (2002).
Pyšek, P., Richardson, D. M. & Williamson, M. Predicting and explaining plant invasions through analysis of source area floras: some critical considerations. Divers. Distrib. 10, 179–187, doi:10.1111/ddi.2004.10.issue-3 (2004).
Hamilton, M. A. et al. Life-history correlates of plant invasiveness at regional and continental scales. Ecol. Lett. 8, 1066–1074, doi:10.1111/j.1461-0248.2005.00809.x (2005).
Castro, S. A., Figueroa, J. A., Muñoz-Schick, M. & Jaksic, F. M. Minimum residence time, biogeographical origin, and life cycle as determinants of the geographical extent of aturalized plants in continental Chile. Divers. Distrib. 11, 183–191, doi:10.1111/ddi.2005.11.issue-3 (2005).
Arroyo, M.T.K., Marticorena, C., Matthei, O. & Cavieres, L.A. Plant invasions in Chile: present patterns and future predictions In Invasive species in a changing world (eds Mooney, H.A. & Hobbs, R.J.) 395–420 (Island, Washington, 2000).
Pauchard, A., Cavieres, L. & Bustamante, R. Comparing alien plant invasions among regions with similar climates: where to from here? Divers. Distrib. 10, 371–375, doi:10.1111/ddi.2004.10.issue-5-6 (2004).
Fuentes, N., Pauchard, A., Sánchez, P., Esquivel, J. & Marticorena, A. A new comprehensive database of alien plant species in Chile based on herbarium records. Biol. Inv. 15, 847–858, doi:10.1007/s10530-012-0334-6 (2013).
Martín-Forés, I. et al. Flora of the Mediterranean basin in the Chilean espinales: evidence of colonization. Pastos 42, 135–158 (2012).
Martín-Forés, I. et al. From Spain to Chile: environmental filters and success of herbaceous species in Mediterranean-climate regions. Biol. Inv. 17, 1425–1438, doi:10.1007/s10530-014-0805-z (2015).
Dainese, M. & Poldini, L. Does residence time affect responses of alien species richness to environmental and spatial processes? NeoBiota 14, 47–66, doi:10.3897/neobiota.14.3273 (2012).
Shea, K. & Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 17, 170–176, doi:10.1016/S0169-5347(02)02495-3 (2002).
Lockwood, J. L., Cassey, P. & Blackburn, T. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 20, 223–228, doi:10.1016/j.tree.2005.02.004 (2005).
Venable, D. L., Burquez, A. M., Corral, G., Morales, E. & Espinosa, F. The ecology of seed heteromorphism on Heterosperma pinnatum in Central Mexico. Ecology 68, 65–76, doi:10.2307/1938805 (1987).
Venable, D. L. & Brown, J. S. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. Am. Nat. 131, 360–384, doi:10.1086/284795 (1988).
Priestley, D. A. Seed aging, implications for seed storage and persistence in the soil (Cornell University Press, New York, 1986).
Baker, H. G. Some aspects of the natural history of seeds banks in Ecology of soil seed banks (eds Leck, M. A., Parker, V. T. & Simpson, R. L.) 9–21 (Academic Press, San Diego, California, 1989).
McEvoy, P. B. Dormancy and dispersal in dimorphic achenes of tansy ragwort Senecio jacobaea. Oecologia 61, 160–168, doi:10.1007/BF00396754 (1984).
Brändel, M. Ecology of achene dimorphism in Leontodon saxatilis. Ann. Bot. 100, 1189–1197, doi:10.1093/aob/mcm214 (2007).
Imbert, E. The effects of achene dimorphism on the dispersal in time and space in Crepis sancta (Asteraceae). Can. J. Bot. 77, 508–513, doi:10.1139/cjb-77-4-508 (1999).
Venable, D. L. & Lawlor, L. Delayed germination and dispersal in desert annuals: escape in space and time. Oecologia 46, 272–282, doi:10.1007/BF00540137 (1980).
Maron, J. L., Elmendorf, S. C. & Vilà, M. Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum. Evolution 61, 1912–1924, doi:10.1111/j.1558-5646.2007.00153.x (2007).
Kumschick, S., Hufbauer, R. A., Alba, C. & Blumenthal, D. M. Evolution of fast-growing and more resistant phenotypes in introduced common mullein (Verbascum thapsus). J. Ecol. 101, 378–387, doi:10.1111/jec.2013.101.issue-2 (2013).
Leishman, M. R., Cooke, J. & Richardson, D. M. Evidence for shifts to faster growth strategies in the new ranges of invasive alien plants. J. Ecol. 102, 1451–1461, doi:10.1111/jec.2014.102.issue-6 (2014).
Sun, Y., Müller-Schärer, H., Maron, J. L. & Schaffner, U. Biogeographic effects on early establishment of an invasive alien plant. Am. J. Bot. 102, 621–625, doi:10.3732/ajb.1400451 (2015).
Imbert, E. Capitulum characters in the seed heteromorphic species Crepis sancta (Asteraceae), variance portioning and inference for the evolution of dispersal rate. Heredity 86, 78–86, doi:10.1046/j.1365-2540.2001.00812.x (2001).
Venable, D. L. The evolutionary ecology of seed heteromorphism. Am. Nat. 126, 577–595, doi:10.1086/284440 (1985).
Sorensen, A. E. Somatic polymorphism and seed dispersal. Nature 276, 174–176, doi:10.1038/276174a0 (1978).
Kigel, J. Diaspore heteromorphism and germination in populations of the ephemeral Hedypnois rhagadioloides (L.) F.W. Schmidt (Asteraceae) inhabiting a geographic range of increasing aridity. Acta Oecologica 13, 45–53 (1992).
Imbert, E. Ecological consequences and ontogeny of seed heteromorphism. Perspect. Plant Ecol. Evol. Syst. 5, 13–36, doi:10.1078/1433-8319-00021 (2002).
Anthos. Sistema de información de las plantas de España. Real Jardín Botánico, CSIC- Fundación Biodiversidad. Electronic source www.anthos.es (accessed 10th of June 2016).
The Plant List. Version 1.1. Published on the Internet; http://www.theplantlist.org/ (accessed 10th of June, 2016).
Groves, R.H. Invasion of Mediterranean ecosystems by weeds In Resilience in Mediterranean-type ecosystems (eds Dell B., Hopkins A.J.M., Lamont B.B.). 129–145 (Dr. W. Junk Publishers, Dordrecht, The Netherlands, 1986).
Ninyerola, M., Pons X, Roure J.M. Atlas Climático Digital de la Península Ibérica. Metodología y aplicaciones en bioclimatología y geobotánica. ISBN 932860-8-7. Universidad Autónoma de Barcelona, Bellaterra. http://opengis.uab.es/wms/iberia/index.htm (2005).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978, doi:10.1002/(ISSN)1097-0088 (2005).
Moloney, K. A., Holzapfel, C., Tielbörger, K., Jeltsch, F. & Schurr, F. M. Rethinking the common garden in invasion research. Persp. Plant Ecol. Evol. Syst. 11, 311–320, doi:10.1016/j.ppees.2009.05.002 (2009).
Burnham, K.P. & Anderson, D.R. Model selection and multimodel inference: A practical information-theoretic approach (2nd edition) (Springer-Verlag, New York, 2002).
Zuur, A.F., Ieno, E.N., Walker, N.J., Saveliev, A.A., Smith, G.M. Mixed effects models and extensions in ecology with R. Statistics for Biology and Health. (Springer-Verlag, New York, USA, 2009).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing (Vienna, Austria, 2015). https://www.R-project.org/.
Bates, D., Maechler, M., Bolker, B., Walker, S., Christensen, R.H.B., Singmann, H. lme 4: Linear mixed-effects models using Eigen and S4. http://cran.r-project.org/web/packages/lme4/index.html. R package version 1,1–6 (2014).
Mazerolle, M.J. AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). http://cran.r-project.org/web/packages/AICcmodavg/index.html. R package 1.35 (2013).
Acknowledgements
We thank the Spanish Ministry of Science and Innovation for the financial support received to carry out this study (CGL2009-08718) and the Spanish Ministry of Education, Culture and Sport, because of the pre-doctoral FPU scholarship of the main author, reference AP2009-0518. We are also grateful for the post-doctoral grant of the main author (REMEDINAL3-CM. S2013/MAE-27 l 9). We thank the State Meteorological Agency for providing meteorological data (AEMET). We are especially grateful for the assistance with the laboratory work given by Devayana Valero, Ricardo Prentice, Lilian Patricia Cáceres and Alejandra Lucía López. M.F.B. was supported by Australian Research Council funding (DE150100542; DP150103414). We would like to acknowledge the whole INIA-Cauquenes Institution, in central Chile, and the team from the Faculty of Agronomy of the Polytechnic University of Madrid.
Author information
Authors and Affiliations
Contributions
I.M.-F. and M.A. carried out the laboratory work; I.M.-F., M.A.C., L.S.-J. and M.F.B. did the data analyses; I.M.F., M.A.C., M.F.B., B.A.-G., I.C. and A.d.P. interpreted results; and I.M.F. wrote the manuscript with assistance from M.F.B., and supervised by B.A.-G., I.C. and M.A.C. J.M.d.M., L.S.-J. and C.O. supervised the work, commented on and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martín-Forés, I., Avilés, M., Acosta-Gallo, B. et al. Ecotypic differentiation and phenotypic plasticity combine to enhance the invasiveness of the most widespread daisy in Chile, Leontodon saxatilis . Sci Rep 7, 1546 (2017). https://doi.org/10.1038/s41598-017-01457-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01457-1
This article is cited by
-
Building trait datasets: effect of methodological choice on a study of invasion
Oecologia (2022)
-
The effect of horticultural trade on establishment success in alien terrestrial true ferns (Polypodiophyta)
Biological Invasions (2021)
-
Functional segregation of resource-use strategies of native and invasive plants across Mediterranean biome communities
Biological Invasions (2021)
-
Shade and nutrient-mediated phenotypic plasticity in the miracle plant Synsepalum dulcificum (Schumach. & Thonn.) Daniell
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.