Abstract
Beta-hemolytic Streptococcus dysgalactiae is a well-known pathogen for a wide range of animals and humans. Two subspecies are recognized: (i) equisimilis, associated to disease in horses and humans, and (ii) dysgalactiae mainly isolated from animal illness with only a few humans’ cases. This study describes the isolation and characterization of Streptococcus dysgalactiae subsp. dysgalactiae (SDSD) from vampire bats, maintained in captivity for research proposes. Animals presented neurologic, respiratory and gastroenteric symptoms and sudden death. Beta-hemolytic Gram-positive cocci were isolated in blood agar plates and further characterized as Lancefield group C. All isolates were identified as S. dysgalactiae by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry and subspecies dysgalactiae was confirmed by 16S rRNA sequencing and phylogenetic analysis. Genotyping through SE-ALFP resulted in three profiles (A1–A3) with one bat being infected by profiles A1 and A3. This is the first report of SDSD causing illness in bats and especially in Desmodus rotundus species.
Similar content being viewed by others
Introduction
The Streptococcus genus comprises Gram-positive, catalase-negative, cytochrome-negative, aerotolerant anaerobe and nonmotile bacteria1,2. The genus is divided into seven groups with Streptococcus dysgalactiae belonging to the pyogenic group2. Most of the pyogenic streptococci are considered pathogenic for humans and animals and are characterized by β-hemolysis due to the activity of hemolysins, streptolysin O and especially streptolysin S. They can also be characterized by polysaccharide variation detectable by the Lancefield method2.
The Streptococcus dysgalactiae is further divided into the subspecies S. dysgalactiae subsp. dysgalactiae (SDSD) and S. dysgalactiae subsp. equisimilis (SDSE)3,4. SDSE includes isolates from human and animals with strong β-haemolysis and is inserted into Lancefield serogroups A, C, G, and L, while SDSD is isolated mainly from animals such as cattle, dogs, pigs and other species3,5,6,7,8,9, with only a couple of human cases associated with articular infection after a total knee arthroplasty10, and cellulitis associated with the preparation of raw seafood11, present α-, β-, or nonhemolytic activity, and belong to Lancefield groups C and L2,3,4.
The Streptococcus genus has been isolated from different bats species12,13,14,15,16. The oral cavity of bats, as other mammals, is colonized by a wide range of streptococcal species mostly belonging to the mutans group12. In rectal swab of four different flying fox species (Pteropus sp.), Heard et al.14 detected α-hemolytic and group D Streptococcus. Helmick et al.15 also associated the presence of α-hemolytic Streptococcus to the death of two captive megachiropteran bats due to pneumonia. The S. dysgalactiae species is poorly studied in bats, even though it has already been isolated from the gut of healthy Desmodus rotundus13.
Here we present the isolation and characterization of Streptococcus dysgalactiae subsp. dysgalactiae from five vampire bats with clinical signs of encephalitis, pneumonia, and sudden death. This is the first report of SDSD causing septicemia and encephalitis in Desmodus rotundus.
Results
Clinical signs
From the 20 Desmodus rotundus that were kept in captive, 18 of them became ill and presented various signs, such as anorexia (1/18), neurologic symptoms (paralysis) (8/18), pneumonia (1/18), and sudden death (8/18), within 13 weeks, on average, of captivity and observation. At post-mortem necropsy, encephalitis and congestion of different organs were observed (Fig. 1). The brain congestion in accordance with the neurologic symptoms presented suggested the possibility of rabies, and also because some cases of rabies in cattle had been reported in the area of capture. All animals were negative for rabies in real-time PCR (data not shown). In attempt to elucidate the causative agent, bacterial isolation was performed from different tissues (lung, liver, intestine, brain) of five bats.
Characteristics of the isolates
Gram-positive, catalase-negative, coccus-shaped organisms were isolated from all clinical samples of different organs suggesting bacteremia. Grey beta-hemolytic colonies were isolated on blood agar after 24 h of incubation at 37 °C. All isolates were characterized as Lancefield group C (Table 1).
MALDI-TOF MS identification
The Streptococcus dysgalactiae species were identified by MALDI-TOF MS for all isolates. Considering this result, 16 S rRNA sequencing and phylogenetic analysis were proceeded for subspecies identification.
16 S rRNA sequencing and phylogenetic analysis
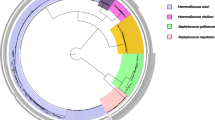
The subspecies identification was obtained through 16 S rRNA sequencing and phylogenetic analysis. All isolates were identified as S. dysgalactiae subspecies dysgalactiae (>99% sequence identity with S. dysgalactiae subsp. dysgalactiae strain ATCC43078) (Fig. 2).
SE-AFLP analysis
SE-AFLP genotyping of the 12 studied isolates resulted in three profiles (A1–A3) with high genetic similarity (>80%) (Fig. 3). The gel with SE-AFLP profiles of SDSD can be found as Supplementary Fig. S1. The A1 cluster comprised eight isolates originated from three animals from different cities, while the A2 profile comprised three strains isolated from two animals of the same city. The 13L isolate presented a distinct fingerprint pattern from the other studied strains, even though it originated from the same animal of the 13Li and 13I isolates (A1 cluster).
Discussion
Streptococci are predominant members of the commensal microbiota of the mucous membranes of the human oral cavity and to a lesser extent of the nasopharynx2. Although some streptococcal commensals have no significant record of disease transgressions, others streptococci have a lot of virulence factors, acting as pathogens capable of spread and of initiating infection in immunocompromised individuals2,9,17. The disease-associated streptococcal species may also occur in the asymptomatic host in which they are maintained in a sub-disease threshold load2. Changes in the pathogen population or to the host environment, such as decrease of competitors, acquisition of virulence factors, immunosuppression, and dietary changes, can affect the host-pathogen relationship and initiate the disease process2,18.
This could explain the observed outbreak, considering that the stress provoked by the environmental changes and animals diet could originate a momentary immunosuppression and favor the pathogen development. As the feeding blood was not contaminated, the S. dysgalactiae was probably introduced by a healthy bat harboring the pathogen, previously to the capture, that started to manifest the disease and excrete the bacteria due to the stressful changes. The horizontal transmission is sustained by the SE-AFLP genotyping that demonstrates high genetic similarity within the studied isolates and also the A1 cluster comprised isolates from three different cities that died in different days.
The symptoms described in the observed bats are common to streptococcal infection and especially to S. dysgalactiae. Also, in captive bats pneumonia has already been related to α-hemolytic Streptococcus infection15. As observed here, sudden death and organ congestion related to SDSD β-hemolytic Lancefield group C infection were also evidenced in puppies6. Streptococcus dysgalactiae is reported as a cause of bacteremia, meningoencephalitis, and mastitis in sheep2, and articular abscess in pigs5. S. dysgalactiae of group C occasionally causes lower limb cellulitis, meningitis, and bacteremia in humans19,20,21. β-hemolytic S. dysgalactiae of groups C and G can cause severe and recurring invasive infections21.
Among the mammalian class, bats are the most important reservoir of zoonotic pathogens and are estimated that they possibly may harbor several “missing zoonoses” that still were not detected22. For this reason, the order of Chiropteran is incriminated as of highest value for surveillance, especially in South and Central America and parts of Asia22. Furthermore, several pathogens that may affect humans such as Bartonella spp23,24., Polyomaviridae25, influenza A virus26, among several others22, and the isolation of pathogenic SDSD shows the probability of transmission of another pathogen by bats to animals and humans. The interface of bats and humans can occur in a variety of ways like incidental contact, degradation of the environment by humans, controlled contact for research proposes and predation27. The widespread distribution of vampire bats on the American continent, ranging from Mexico to southern South America22,28,29, encompasses around fourteen countries29 and shows that millions of people and animals are vulnerable to bats attacks in rural areas and also in places where the degradation of the environment alters the proximity between human and animals, and those scenarios are very common in the geographic areas where the vampire bat is found.
The S. dysgalactiae species identification was obtained through MALDI-TOF MS and subspecies dysgalactiae was only identified by 16 S rRNA sequencing and phylogenetic analysis. Even though MALDI-TOF MS has comparable discriminatory power to molecular techniques for bacterial species differentiation30 and presented high-confidence for β-hemolytic streptococci species identification31, this technique still does not enable proper S. dysgalactiae subspecies differentiation. For subspecies identification, the phenotypic characterization still causes confusion, as corroborated by our results since Lancefield group C β-hemolytic colonies could be allocated in both subspecies. Considering that the misclassification of β-hemolytic SDSD into subspecies equisimilis has been previously reported4,6 and was only solved by 16S rRNA analysis, the molecular techniques still are more appropriate for this differentiation level of S. dysgalactiae. Interestingly, Jensen & Kilian4 observed that all β-hemolytic SDSD originated from invasive infections, which also corroborates our results.
We present the first report of SDSD infection in vampire bats (Desmodus rotundus) causing septicemia and encephalitis. Even though the clinical relevance of SDSD for animal health further increases, its zoonotic potential remains unknown. Therefore, due to the importance of S. dysgalactiae subspecies, they should be properly identified by veterinary diagnostic laboratories. Also, the presence of SDSD in bats corroborates with the theory that bats harbor a lot of pathogens, which raises the question about the possible involvement of this species in the dissemination of this and others pathogens.
Methods
Animals
The study was designed for the study of serological evaluation of anti-rabies antibodies in bats as an epidemiological tool for the control of the disease in hematophagous chiropterans. Twenty Desmodus rotundus were kept in captive (authorization number 51231-1, issued by the Ministry of the Environment of Brazil) for research proposes. The bats were captured from natural caverns in three nearby cities from São Paulo state (Anhembi, Botucatu, and Bofete) (Table 1) and were considered as asymptomatic. The bats were housed and handled following the ethical principles adopted by Bioethics Commission of the Faculty of Veterinary Medicine and Animal Production of São Paulo State University (protocol number 85/2015) and all experimental protocols were approved by the same institution. Animals were fed with defibrinated blood that was collected in slaughterhouses, supplemented with cobalamin. The bats were observed for 137 days and within 85 days of the quarantine period, on average, 18 of them became ill presenting various signs (Table 1).
Bacterial isolation and Lancefield group determination
Samples from different tissues (lung, liver, intestine, brain) of five bats were used in an attempt for bacterial isolation. The samples were inoculated on blood agar plates (CM0055, Oxoid, Hampshire, England) supplemented with 5% defibrinated sheep blood and incubated aerobically for 24 h at 37 °C. For isolated colonies, the Lancefield group was determined through commercial latex agglutination kit (Avipath Strep®, Omega Diagnostics, Scotland, United Kingdom) following manufacturer’s instructions. Samples of the blood used in the bats feeding were also inoculated on blood agar plates; however, no isolation was obtained.
MALDI-TOF mass spectrometry identification
The obtained isolates were initially identified as Streptococcus dysgalactiae by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry. MALDI-TOF MS sample preparation, data processing, and analysis were done as previously described by Hijazin et al.30. Mass spectra were acquired by Microflex™ mass spectrometer (Bruker Daltonik), with a mass range of 2–20 kDa, using flexControl™ 3.0 software (Bruker Daltonik). Spectra were loaded into MALDI BioTyper™ 3.0 (Bruker Daltonik), using default settings, and compared with the manufacturer’s library. Standard Bruker interpretative criteria were applied; scores ≥ 2.0 were accepted for species assignment and scores ≥1.7 but ≤2.0 for identification at the genus level.
16S rRNA sequencing and phylogenetic analysis
The subspecies identification was achieved through 16 S rRNA sequencing (1–1.3 kb) and phylogenetic analysis. DNA extraction was performed according to Boom et al.32 protocol with previous enzymatic treatment with lysozyme (100 mg) and proteinase K (20 mg) (US Biological, Swampscott, MA, USA) at 37 °C for 60 min. The 16 S rRNA gene amplification was performed using Twomey et al.33 primers. The Illustra GFXTM PCR DNA and Gel Band Purification kit (GE Healthcare) was used for amplicon purification and sequencing was performed by the Human Genome Research Center (Universidade de São Paulo, Brazil). The phylogenetic analysis was performed with Mega 5.10 software using the maximum-likelihood method, and 1000 bootstrap replicates were used for branch support statistical inference. All DNA sequences from this study were deposited in GenBank under accession numbers MF113276 - MF113287.
SE-AFLP genotyping
The obtained isolates were further genotyped by single enzyme amplified fragments length polymorphism (SE-AFLP) following McLauchlin et al.34 protocol. DNA fragments were detected by electrophoresis at 24 V for 26 h in 2% agarose gel stained with BlueGreen® (LGC Biotecnologia, São Paulo, Brazil). Fingerprint patterns were analyzed by comprehensive pairwise comparisons using Dice coefficient. A dendrogram was generated by Bionumerics 7.6 (Applied Maths, Saint-Martens-Latem, Belgium) and a 90% genetic similarity cut-off value was applied for cluster analysis35.
Change history
19 July 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Schaechter, M., Ingraham, J. L. & Neidhardt, F. C. In Microbe (eds Schaechter, M., Ingraham, J. L. & Neidhardt, F. C.) Chapter 6, 82–111 (ASM Press, 2006).
Tagg, J., Wescombe, P. & Burton, J. In LacticAcid Bacteria(eds Von Wright, A., Seppo, S., Ouwehand, A. C. & Lahtinen, S.) 123–146 (CRC Press, 2011), https://doi.org/10.1201/b11503-8.
Vandamme, P., Pot, B., Falsen, E., Kersters, K. & Devriese, L. A. Taxonomic study of lancefield streptococcal groups C, G and L (Streptococcus dysgalactiae) and proposal of S. dysgalactiae subsp. equisimilis subsp. nov. Int. J. Syst. Bacteriol. 46, 774–81 (1996).
Jensen, A. & Kilian, M. Delineation of Streptococcus dysgalactiae, its subspecies, and its clinical and phylogenetic relationship to Streptococcus pyogenes. J. Clin. Microbiol. 50, 113–26 (2012).
Moreno, L. Z. et al. Molecular and antimicrobial susceptibility profiling of Streptococcus dysgalactiae isolated from swine. Diagn. Microbiol. Infect. Dis. 86, 178–80 (2016).
Vela, A. I. et al. Neonatal mortality in puppies due to bacteremia by Streptococcus dysgalactiae subsp. dysgalactiae. J. Clin. Microbiol. 44, 666–668 (2006).
Zadoks, R. N., Middleton, J. R., McDougall, S., Katholm, J. & Schukken, Y. H. Molecular epidemiology of mastitis pathogens of dairy cattle and comparative relevance to humans. J. Mammary Gland Biol. Neoplasia 16, 357–372 (2011).
Abdelsalam, M., Eissa, A. E. & Chen, S. C. Genetic diversity of geographically distinct Streptococcus dysgalactiae isolates from fish. J. Adv. Res. 6, 233–238 (2013).
Janda, W. M. The Genus Streptococcus – Part I: Emerging Pathogens in the “Pyogenic Cocci” and the “Streptococcus bovis” Groups. Clin. Microbiol. Newsl. 36, 157–166 (2014).
Park, M. J. et al. Streptococcus Dysgalactiae Subspecies Dysgalactiae Infection after Total Knee Arthroplasty: A Case Report. Knee Surg. Relat. Res. 24, 120–123 (2012).
Koh, T. H. et al. Streptococcal cellulitis following preparation of fresh raw seafood. Zoonoses Public Health 56, 206–8 (2009).
Takada, K. & Hirasawa, M. Streptococcus dentirousetti sp. nov., isolated from the oral cavities of bats. Int. J. Syst. Evol. Microbiol. 58, 160–163 (2008).
Chaverri, G. Flora bacteriana aeróbica del tracto digestivo del vampiro común, Desmodus rotundus (Chiroptera_Phyllostomidae). Rev. Biol. Trop. (Int. J. Trop. Biol.) 54, 717–724 (2006).
Heard, D. J., De Young, J. L., Goodyear, B. & Ellis, G. A. Comparative rectal bacterial flora of four species of flying fox (Pteropus sp.). J. Zoo Wildl. Med. 28, 471–5 (1997).
Helmick, K. E. et al. A Pasteurella-like bacterium associated with pneumonia in captive megachiropterans. J. Zoo Wildl. Med. 35, 88–93 (2004).
Borda, D., Năstase-Bucur, R., Spînu, M., Uricariu, R. & Mulec, J. Aerosolized Microbes from Organic Rich Materials: Case Study of Bat Guano from Caves in Romania. J. Cave Karst Stud. 76, 114–126 (2014).
Watanabe, S., Takemoto, N., Ogura, K. & Miyoshi-Akiyama, T. Severe invasive streptococcal infection by Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis. Microbiol. Immunol. 60, 1–9 (2016).
Mosier, D. A. Bacterial pneumonia. Vet. Clin. North Am. Food Anim. Pract. 13, 483–93 (1997).
Wajima, T. et al. Molecular Characterization of Invasive Streptococcus dysgalactiae subsp. equisimilis, Japan. Emerg. Infect. Dis. 22, 247–54 (2016).
Rantala, S. Streptococcus dysgalactiae subsp. equisimilis bacteremia: an emerging infection. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1303–1310 (2014).
Trell, K., Sendi, P. & Rasmussen, M. Recurrent bacteremia with Streptococcus dysgalactiae: a case-control study. Diagn. Microbiol. Infect. Dis. 85, 121–4 (2016).
Olival, K. J. et al. Host and viral traits predict zoonotic spillover from mammals. Nature 546, 646–650 (2017).
Bai, Y. Bartonella spp. in Bats, Guatemala. Emerg. Infect. Dis. 17, 1269–1272 (2011).
Veikkolainen, V., Vesterinen, E. J., Lilley, T. M. & Pulliainen, A. T. Bats as Reservoir Hosts of Human Bacterial Pathogen, Bartonella mayotimonensis. Emerg. Infect. Dis. 20, 960–967 (2014).
Tao, Y. et al. Discovery of diverse polyomaviruses in bats and the evolutionary history of the Polyomaviridae. J. Gen. Virol. 94, 738–48 (2013).
Tong, S. et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. 109, 4269–4274 (2012).
Wood, J. L. N. et al. A framework for the study of zoonotic disease emergence and its drivers: spillover of bat pathogens as a case study. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 367, 2881–92 (2012).
Greenhall, A. M., Joermann, G., Schmidt, U. & Seidel, M. R. Desmodus rotundus. Mamm. Species 1, https://doi.org/10.2307/3503895 (1983).
Johnson, N., Aréchiga-Ceballos, N. & Aguilar-Setien, A. Vampire Bat Rabies: Ecology, Epidemiology and Control. Viruses 6, 1911–1928 (2014).
Hijazin, M. et al. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) for species identification of bacteria of genera Arcanobacterium and Trueperella. Vet. Microbiol. 157, 243–245 (2012).
Cherkaoui, A., Emonet, S., Fernandez, J., Schorderet, D. & Schrenzel, J. Evaluation of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Rapid Identification of Beta-Hemolytic Streptococci. J. Clin. Microbiol. 49, 3004–3005 (2011).
Boom, R. et al. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28, 495–503 (1990).
Twomey, D. F., Carson, T., Foster, G., Koylass, M. S. & Whatmore, A. M. Phenotypic characterisation and 16S rRNA sequence analysis of veterinary isolates of Streptococcus pluranimalium. Vet. J. 192, 236–8 (2012).
McLauchlin, J., Ripabelli, G., Brett, M. M. & Threlfall, E. J. Amplified fragment length polymorphism (AFLP) analysis of Clostridium perfringens for epidemiological typing. Int. J. Food Microbiol. 56, 21–8 (2000).
van Belkum, A. et al. Guidelines for the validation and application of typing methods for use in bacterial epidemiology. Clin. Microbiol. Infect. 13, 1–46 (2007).
Acknowledgements
To Dr. Teruê Sadatsune from the Laboratório de Bacteriologia Médica of Universidade Estadual Paulista “Júlio de Mesquita Filho” for her collaboration. This study was supported by CAPES and CNPq research grants. A.M.M., M.B.H., and J.M. are CNPq fellows. L.Z.M. is FAPESP fellow (Process 2016/25745-7).
Author information
Authors and Affiliations
Contributions
Mioni, M.S.R. performed microbiological isolation and wrote the main manuscript; F.F.C. Castro performed the capture and necropsy of Desmodus rotundus bats; L.Z. Moreno was responsible for molecular techniques and contribution to the conception of the main manuscript; C.M. Apolinário, L.D. Belaz, M.G. Peres, B.L.D. Ribeiro, M.J.S. Castro, A.M. Ferreira, A. Cortez, A.M. Moreno, M.B. Heinemann, and J. Megid were involved in several aspects of the research design and development. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mioni, M.d.S.R., Castro, F.F.C., Moreno, L.Z. et al. Septicemia due to Streptococcus dysgalactiae subspecies dysgalactiae in vampire bats (Desmodus rotundus). Sci Rep 8, 9772 (2018). https://doi.org/10.1038/s41598-018-28061-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-28061-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.